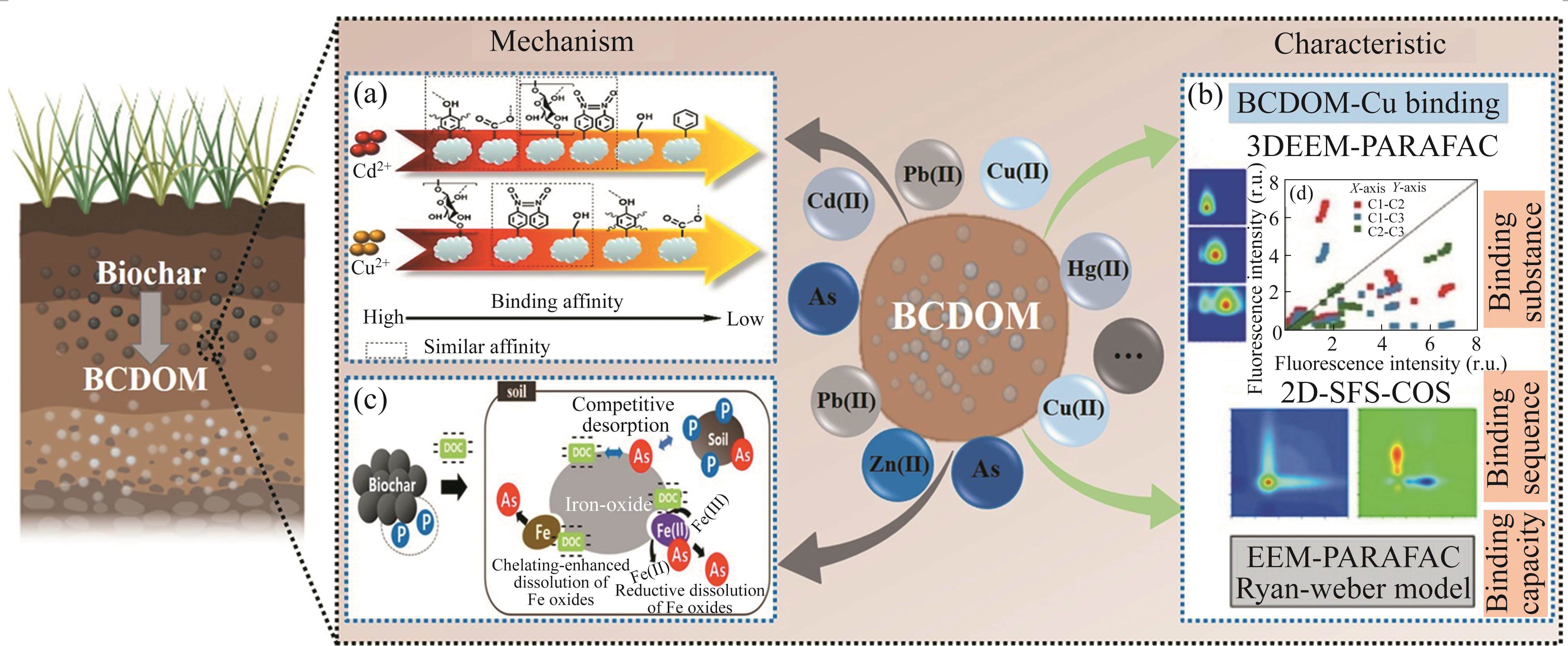
1 Introduction
Environmental pollution is an increasingly serious problem that has attracted widespread attention. In particular, soil pollution caused by heavy metals and organic contaminants has increased dramatically over the last 30 years [1-5]. Therefore, to ensure sustainable agricultural development, it is urgent to search for a cost-effective and environmentally friendly approach to reduce soil pollution. Biochar, also known as bio-coal, is generally produced by pyrolysis of natural and waste biomass under an inert atmosphere at a wide range of temperatures. Due to its unique physicochemical properties, biochar has received much attention over the past two decades for its ability to modify soil properties [6-8], clean fuel production [9, 10], waste management strategies [11-14], environmental remediation [15-17], and climate change mitigation [18, 19]. Previous research has indicated that biochar can immobilize heavy metals and organic contaminants, thereby reducing soil pollution [20-25]. One study indicated that after the application of multiple-modified biochar, the extractable Cu(II) and Cd(II) were decreased by 100% and 92.0% for farmland soil, and 100% and 90.3% for vegetable soil, respectively [26]. In addition, the application of biochar to soil could promote soil bacteria growth, supply nutrients, and modify soil structure and properties, including soil pH, organic carbon, available phosphorous and potassium, electrical conductivity, and water retention, thus increasing crop yields [27-29].
Due to the above advantages, biochar has been favored by a wide range of research in the field of soil remediation in recent years [25, 30]. When biochar is applied to the soil, it can release dissolved organic matter (BCDOM) into the soil environment under the conditions of irrigation or natural rainfall, thus changing the properties of the soil by binding soil particles together, leading to better water retention, lower erosion, and higher soil porosity [31, 32]. Generally, BCDOM is composed of organic molecules that can pass through a 0.45 μm filter, including humic biopolymers, hydrophobic organic carbon, and some acids with low molecular weight [33-36]. Although BCDOM has a small proportion in biochar (0.17-37.03 (mg/g)), it has received noticeable attention as an essential part of biochar and plays an important role in the application of biochar for soil remediation [13, 37, 38]. Because of the abundance of functional groups, BCDOM can interact with some soil contaminants including heavy metals, organic pollutants, and biologicals, which can alter the transport and fate of BCDOM and soil pollutants [39-41]. In addition, BCDOM can serve as a food source for soil microorganisms, stimulating microbial activity and increasing soil fertility and nutrient cycling, thereby improving the soil remediation capacity of biochar [42, 43]. Relevant researches have proved that BCDOM not only provides essential nutrients for plant growth [44, 45] but also enhances plant disease resistance, promoting plant growth [46-48]. The explanation for this phenomenon is that several proteins, generated by BCDOM and biochar, are involved in stimulating plant growth [49]. As a result, a great deal of attention and interest have been paid to the topic of biochar and BCDOM applications in soil remediation.
However, the factors affecting the nature of BCDOM and its interaction process and mechanism with soil contaminants, including organic pollutants and heavy metals, are still unclear. Therefore, an in-depth understanding of BCDOM properties and the mechanism of its interaction with pollutants is essential to realize efficient and accurate application of biochar in soil ecosystems. To the best of our knowledge, there are few reviews to investigate the effects of BCDOM on soil contaminants and their interaction mechanisms in the soil environment. To better understand the roles and effects of BCDOM on the soil environment, as well as to better utilize the application of biochar in soil remediation, a systematic review of this issue is necessary. Based on the analysis and summarization of the literature, the objective of this review is to: 1) summarize the influencing factors of BCDOM generation; 2) discuss the characterization methods of BCDOM; 3) summarize the interaction between BCDOM and soil heavy metals, organic contaminants, soil microorganisms, and plants; 4) analyze the transformation of BCDOM in the soil system; and 5) discuss the challenges and further research directions of BCDOM. By summarizing and analyzing the literature, this work will provide scientific insights and a better understanding of the application of biochar and BCDOM.
2 Factors influencing BCDOM properties
Generally, a certain amount of BCDOM can be released during biochar application to soil, which is mainly governed by the inherent characteristics of biochar and the external extraction methods. It is well known that the biochar’s properties largely depend on a variety of factors, such as feedstocks, preparation temperatures, and preparation technologies. As a result, the composition, quantity, and properties of BCDOM also depend on the biochar preparation methods and extraction conditions (Figure 1).
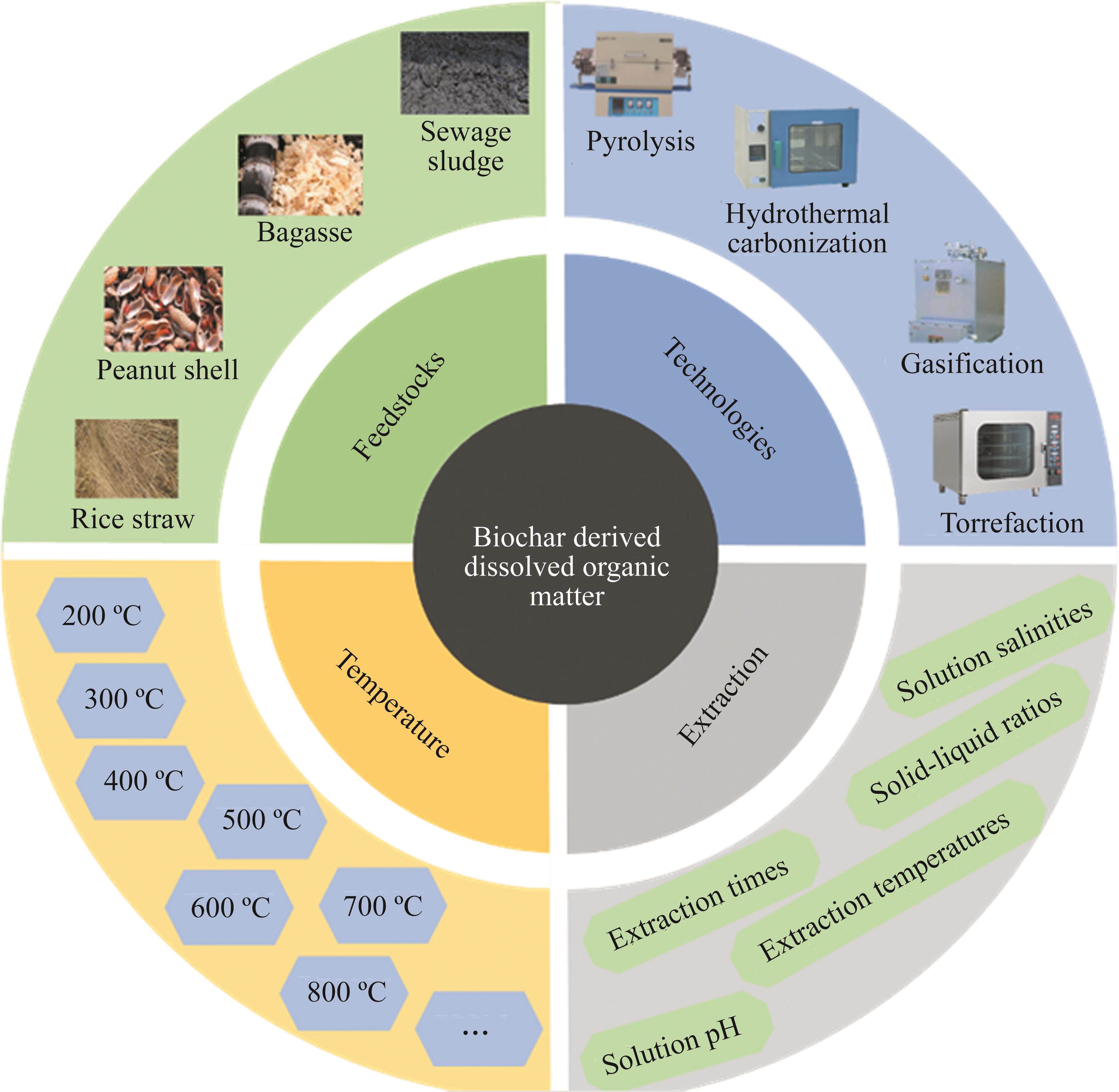
2.1 Internal influencing factors
The internal factors affecting BCDOM primarily include feedstock, carbonization method, and pyrolysis temperature of biochar. In particular, the feedstock for biochar preparation is an important factor affecting the quantity and composition of BCDOM [33, 50, 51]. Up to now, most studies focused on BCDOM extracted from biochar prepared by plants and animal manure [1, 31, 52, 53]. In general, biochars prepared from manure and sawdust feedstocks produce a higher amount of BCDOM than those prepared from sludge and straw feedstock [54]. Studies revealed that BCDOM extracted from agricultural residues biochar ranged from 0.435 to 37.03 mg/g [55], while the content of BCDOM derived from livestock manure biochar ranged from 0.17 to 25 mg/g [31]. TANG’s group found that the concentration of dissolved organic carbon (DOC) in five biochar produced from different feedstock, such as rice straw, sewage sludge, peanut, mushroom residue, and soybean straw, ranged from 17.6 to 53.8 mg/L, which was ascribed to the different properties of the biochar feedstocks [56]. KIM et al [57] found that BCDOM content tended to increase with decreasing the lignin content in the feedstock of wood chips, manifesting that the content of lignin played a decisive role in controlling the BCDOM release from biochar. The inverse relationship between BCDOM leaching and lignin content might be explained by the fact that the higher the lignin content in the feedstock, the more non-biodegradable aromatic compounds in the biochar, leading to the more stable structure of the biochar [33, 58]. Additionally, the composition of BCDOM derived from biochar prepared by different feedstocks is remarkably different. Generally, BCDOM derived from manure-based biochar had a higher content of humic acid-like substance [38]. The content of protein-like and humic acid-like substances in BCDOM derived from N-rich sludge was higher than that of C-rich feedstocks (i.e., walnut, pine needle, and wheat straw), suggesting that the BCDOM derived from N-rich sludge was more biologically labile than C-rich feedstocks [59]. In another study, the relative abundance of humic acid-like substances in garlic stem biochar was significantly higher than that of biochar prepared from perilla, soybean straw, tea waste, rice husk, oak wood, and pine wood chips [51]. Through the identification of fluorescence spectral indicators, LIU’s team found that the humification degree of BCDOM extracted from macroalgal-based biochar was higher than that of other biochar, indicating that the composition and distribution of BCDOM released from biochar varied remarkably with feedstock [60]. Consequently, the selection of lignin-rich and N-rich biomass feedstock is an important factor in improving the content of protein-like and humic acid-like substances in BCDOM.
The preparation technologies of biochar mainly include hydrothermal carbonization, pyrolysis, gasification, and torrefaction, which convert biomass into biochar under different temperature conditions [61-63]. Pyrolysis is a process for converting biomass into biochar and some by-products under oxygen-free conditions at 300-900 ℃ [64, 65]. However, for biomass with high moisture quantity, hydrothermal carbonization is more suitable than pyrolysis because it is performed at relatively low temperatures and water pressure [66-68]. Generally, the biochar produced by pyrolysis exhibits relatively high thermal stability and abundant aromatic structures, while a large number of aliphatic structures exist in hydrochar [69]. SONG’s group assessed the DOM released from hydrochar and biochar prepared from pig manure under various conditions [38]. The content of BCDOM extracted from biochar and hydrochar ranged from 0.38% to 0.48% and 3.34% to 11.96%, respectively. Moreover, compared with BCDOM, the hydrochar-DOM exhibited lower aromaticity and larger molecular weight, indicating that the types of organic functional groups in DOM extracted from hydrochar were higher than those of BCDOM [70]. In addition, research revealed that the hydrochar-DOM mainly consists of humic acid- like substances, phenolic, and less aromatic structures [71]. The BCDOM components, however, consisted mainly of humic acid-like, tyrosine-like, and fulvic acid-like substances [72]. Interestingly, it was found that the relative abundance of tannins-like, protein-like, condensed aromatic, carbohydrates-like, and unsaturated hydrocarbons structures in BCDOM increased, while lipid-like and lignin-like structures showed a decreasing trend after biochar treatment with thermal air oxidation [73]. After the aging treatment, the BCDOM content of biochar treated with H2O2-rich soil solution was 147.26%-734.13% higher than the control samples [74]. Therefore, the preparation of biochar by hydrothermal method and proper secondary treatment is more beneficial to increase the content of BCDOM and organic functional groups of BCDOM.
Carbonization temperature has been recognized as the main factor affecting the properties of biochar. Hence, the quantity and composition of BCDOM might also be influenced by the carbonization temperatures of biochar [59, 75]. Generally, the quantity of BCDOM released from biochar at low pyrolysis temperatures is higher than that at high carbonization temperatures [2, 50, 76]. The reason might be explained by the fact that high carbonization temperature reduces the quantity of volatile matter and increases the content of fixed carbon in biochar, resulting in a decline in the quantity of BCDOM [77-79]. For instance, BIAN’s group reported that the fraction of BCDOM extracted from wheat straw biochar pyrolyzed at 350 ℃ was higher than that of biochar at 450 and 550 ℃ in terms of organic matter content, organic molecule abundance, and mineral nutrients [46]. Another study found that when the carbonization temperature was increased from 200 to 500 ℃, the content of DOC extracted from chicken, swine, and dairy manure biochar decreased sharply from 25.0, 16.6 and 10.9 mg/g to 0.17, 0.33 and 0.21 mg/g, respectively. As the carbonization temperature was further increased, the DOC content remained unchanged, indicating that the DOC content in the biochar reached a stable level [31]. In addition, LIU’s team [60] found that the bioavailability of BCDOM extracted from macroalgae biochar was higher at pyrolysis temperature of 200-300 ℃ than at 400-500 ℃. Therefore, biochar prepared at a lower carbonization temperature is more likely to obtain a higher amount of BCDOM.
With the variation of carbonization temperature, the composition of BCDOM as well as the content of each component will alter. In general, fulvic acid-like, humic acid-like, and protein-like fractions are the major components of BCDOM [60, 80, 81]. Studies have confirmed that fulvic acid-like fractions were the primary components of BCDOM at the pyrolysis temperature of 300-500 ℃, while BCDOM was constituted by humic acid-like components at 500-700 ℃ without considering the feedstock [72, 77, 80]. LI et al [55] revealed that the relative proportion of humic acid-like components in BCDOM derived from agricultural residues biochar was enhanced at high temperatures, which account for 55%-78%. In WEI et al’s study [2], the relative ratio of humic acid-like components associated with aromatic structures increased with increasing pyrolysis temperature, leading to an increase of BCDOM extracted from Jerusalem artichoke stalks biochar. Moreover, the aromatic-C species account for 31.0%-34.2% of the total C in BCDOM. Similar results were also reported by other researches [51, 82]. Therefore, it could be concluded that the molecular weight and aromaticity of BCDOM were high at high carbonization temperatures. However, at relatively high temperatures, HE et al’s groups [50] discovered that protein-like fraction, including tyrosine and tryptophan, was dominant in the BCDOM derived from fruit peel, sludge, and human manure biochar. A possible reason for this phenomenon is the difference in feedstocks. Therefore, it can be inferred that the selection of feedstocks with high lignin content and the preparation of biochar by hydrothermal method or lower carbonization temperature are more conducive to the release of BCDOM with a high content of fulvic acid-like fractions.
2.2 External influencing factors
Previous studies have proved that the quantity and composition of BCDOM are not only related to the biochar preparation process, but also rely on the extraction conditions, including extraction temperatures, pH and salinity of the extraction solution, extraction times, and solid-to-liquid ratios [56, 80, 83, 84]. Currently, there is no standard methodology for extracting BCDOM from biochars. In general, the quantity of BCDOM gradually increases as the extraction solution pH increases, whereas the influence of solution salinity on the content of BCDOM is highly dependent on the nature of the biochar [55, 85]. When the extraction solution was NaOH (0.1 mol/L), the total content and concentration of DOC released from P. australis biochar were 23.5 and 1.22 g/L, respectively, which were noticeably higher than those of HCl, deionized water, and NaCl solution (Figure 2(a)) [85]. The reason for this phenomenon is that the release and hydrolysis reactions of several oxygen-rich functional groups on the surface of biochar, such as alcohols, carboxyl, aliphatic ethers, are enhanced by alkaline extraction [60, 86, 87]. As displayed by the fluorescence intensity in Figure 2(b), the humic acid-like (C1, C3 and C4) and fulvic acid-like components (C2) were significantly impacted by the extraction conditions, acidic extraction conditions promoted the release of humic acid-like components, especially C1 and C3. According to the relative distribution of the components (Figure 2(c)), it was possible to conclude that humic acid-like substances were the main composition of BCDOM in both acidic solution and deionized water, whereas humic acid-like and fulvic acid-like components predominated in alkaline and salt extraction solutions, respectively [55].
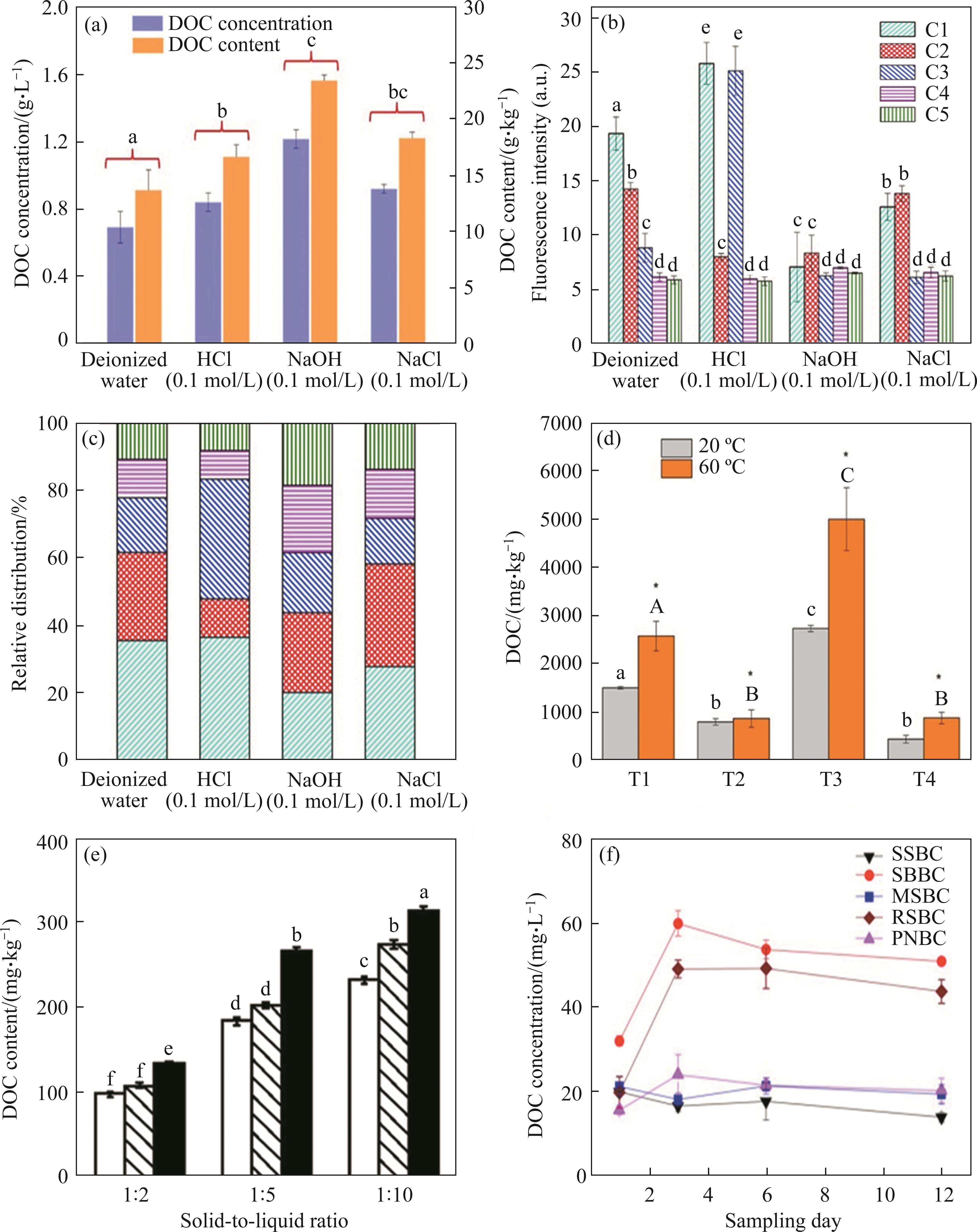
Additionally, the extraction temperature also clearly influences the content and composition of BCDOM. The amount of DOC and humic acid-like substances extracted from wheat straw biochar was higher at 60 ℃ than at 20 ℃ (Figure 2(d)) [55]. This difference was attributed to the fixed carbon content of biochar (i.e., phenols, proteins, and carbohydrates) being more readily desorbed under thermal conditions, which increased the solubility of BCDOM [88, 89]. Another key factor in regulating the quantity and composition of BCDOM is the solid-to-liquid ratio. It was reported that the total content of BCDOM compositions was increased by 2.2 and 0.6 times when the solid-to-liquid ratio was reduced from 1:2 to 1:10 (Figure 2(e)) [90]. The DOC concentrations extracted from soybean straw biochar and rice straw biochar increased significantly at the beginning of extraction (1 to 3 d), but then showed a decreasing trend after 3 d (Figure 2(f)) [56]. As the extraction times increased from 10 to 300 min, the DOC content in vegetable soil, forest soil, and paddy soil increased by 0.8, 1.0 and 1.2 times, respectively [90]. These results distinctly manifest that prolongation of extraction time exhibits a promoting influence on the quantity of BCDOM. Consequently, it is essential to find an appropriate approach for BCDOM extraction. Table 1 displays the properties of BCDOM produced from different feedstocks, preparation technologies, and extraction conditions.
| Feedstock | Preparation method | Extraction condition | Properties of BCDOM | Reference |
|---|---|---|---|---|
| Wheat straw | Pyrolysis (500 ℃) | Deionized water (pH 5.5-7.0), HCl (0.1 mol/L, pH 1.0), NaOH (0.1 mol/L, pH 13.0), and NaCl (0.1 mol/L, pH 7.0). | Humic acid-like and fulvic acid-like substances were the primary components of BCDOM. | [55] |
| Pig manure | Hydrothermal carbonation (170, 180 and 190 ℃) and pyrolysis (200, 300, 400 and 500 ℃) | Distilled water | The concentration of DOM in hydrochar was higher than that of biochar. The protein-like material in the hydrochar-DOM was more abundant than that of BCDOM. The aromaticity of the hydrochar-DOM was lower than that of BCDOM. | [38] |
| Wheat straw | Pyrolysis (350, 450 and 550 ℃) | Hot distilled water | The content of organic matter and mineral materials of BCDOM extracted from biochar at 350 °C was higher than that of biochar at 450 and 550 ℃. | [46] |
| Wetland plant (Typha orientalis) | Pyrolysis (300-700 ℃) | Deionized water | The quantity of BCDOM extracted from low temperatures biochars (300-500 ℃) was higher than that of high temperatures(600-700 ℃). BCDOM was mainly composed of fulvic acid-like substances at low pyrolysis temperatures, while humic acid-like components were the key substances of BCDOM at high temperatures. | [72] |
| Wetland plant (australis) | Pyrolysis (400 ℃) | Deionized water, HCl, NaOH, and NaCl solution (0.1 mol/L, respectively) | The quantity of BCDOM was the highest in the alkaline extraction solution. In water and HCl solution, humic acid-like components were the main components of BCDOM. In alkaline and salt solutions, fulvic acid-like and humic acid-like components were the main BCDOM components. | [85] |
| Chicken, swine, and dairy manure | Pyrolysis (200-700 ℃) | Deionized water | The low temperature was beneficial to obtain higher BCDOM content. BCDOM was mainly composed of protein-like and humic acid-like components. | [31] |
| to be continued | ||||
3 Characterizations of BCDOM
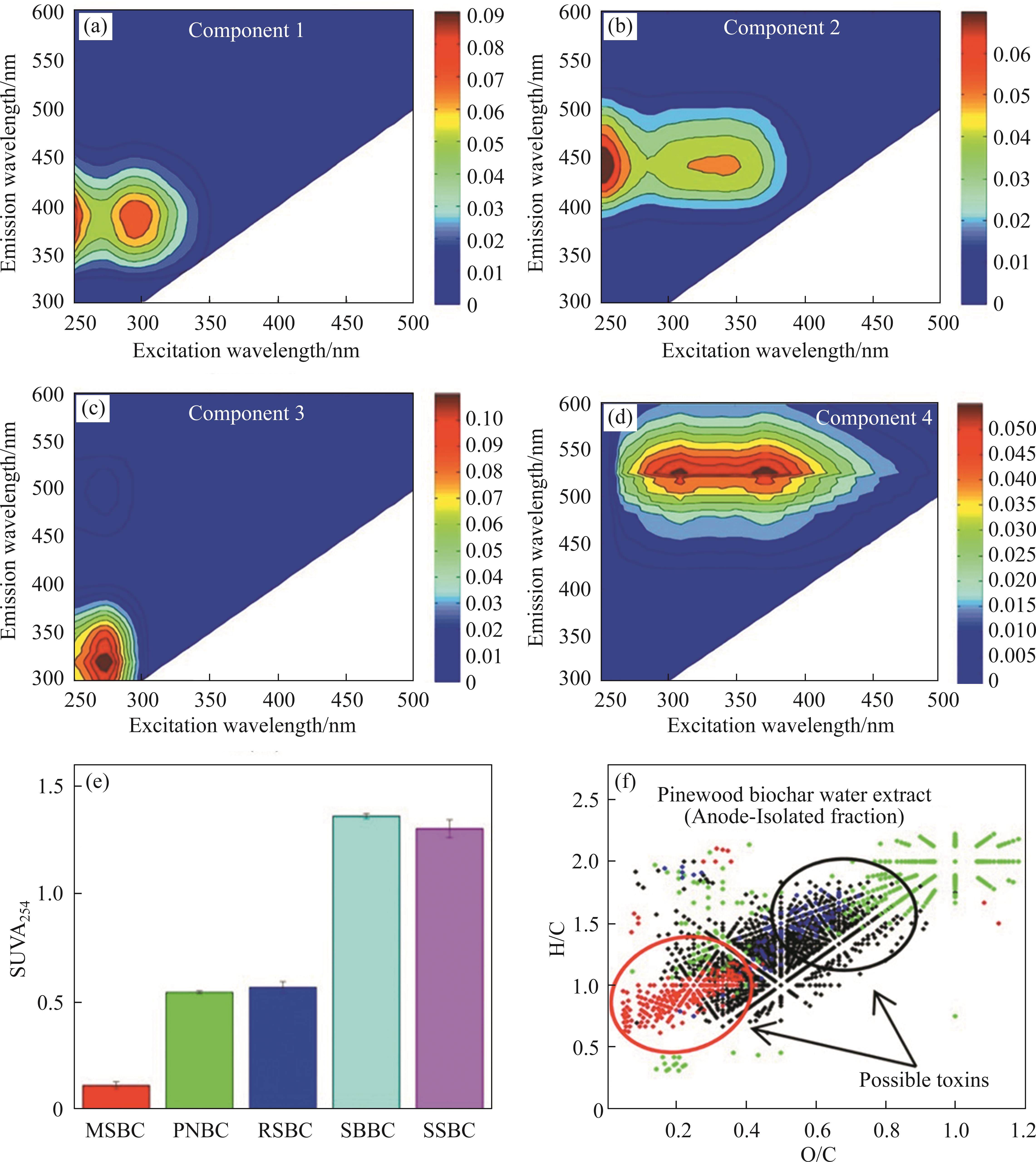
The composition, quantity, and structure of BCDOM vary widely depending on the biochar feedstock, preparation methods, and extraction methods [31, 60, 81, 85, 96]. To better understand BCDOM, a series of technologies have been utilized to investigate the properties of BCDOM [35]. To characterize BCDOM qualitatively and quantitatively, fluorescence excitation-emission matrix spectroscopy coupled with parallel factor analysis (EEM-PARAFAC) and synchronous fluorescence spectra combined with two-dimensional correlation spectroscopy (2D-SFS-COS) has been utilized frequently [56, 97, 98]. According to the peak location, JAMIESON et al [82] found that there were four fluorescent components in BCDOM, among which C1, C2 and C4 fluorophores were typical humic acid-like substances, and C3 fluorophore was identified as protein-like substances (e.g., tyrosine and tryptophan) (Figures 3(a)-(d)). Generally, the specific UV-vis absorbance (SUVA254) is an indicator representing the aromatization degree of BCDOM, which is a normalized value of the absorbance coefficient of BCDOM at 254 nm [37, 99]. Higher values of SUVA254 (SUVA254>4) indicate a higher content of aromatic and strongly hydrophobic substances in BCDOM [94, 100]. As shown in Figure 3(e), the SUVA254 values of all BCDOM samples were lower than 3, indicating that these BCDOM extracted from different biochars were mainly composed of hydrophilic materials [56, 101]. In addition, the absorption values of BCDOM at 260 nm (SUVA260) and 280 nm (SUVA280) were estimated as the proportion of hydrophobic components and the molecular weight of organic components, respectively [102].
| Feedstock | Preparation method | Extraction condition | Properties of BCDOM | Reference |
|---|---|---|---|---|
| Wheat straw, pine needle, alfalfa, walnut shells, sludge, and pig manure | Pyrolysis (300, 500 and 700 ℃) | Distilled water | BCDOM has the highest aromaticity at 700 ℃. BCDOM contained more polycyclic aromatics substances and less protein-like and humic acid-like components. | [59] |
| Cotton stalk | Pyrolysis (600 ℃) and thermally oxidized (350 ℃) | Distilled water | After thermally oxidized, BCDOM had a lower molecular weight, lower O/C ratio, and H/C ratio. Lignin-like components were the main substances of BCDOM. | [73] |
| Pinewood | Pyrolysis (500 and 700 ℃) | Distilled water | BCDOM derived from biochar at 700 ℃ has more aromatic structures. The BCDOM derived from biochar at 700 ℃ mainly contained aromatic protein II, humic acid-like, and fulvic acid-like components, whereas the BCDOM derived from biochar at 500 ℃ mainly contained humic acid-like components. | [91] |
| Corn straw | Pyrolysis (600 ℃) | Corn straw was mixed with red, yellow, and brown soil for 30th, 50th, 70th, 90th and 120th days, respectively. | The aromaticity of BCDOM exhibited a trend of first increasing and then reducing as the extension of mixing time. | [92] |
| Peanut hulls and corn stalks | Pyrolysis (500 ℃) | Deionized water | Humic acid-like components were the major substances of both peanut hulls BCDOM and corn stalks BCDOM. Corn stalks BCDOM contained more protein-like and fulvic acid-like components than peanut hulls BCDOM. | [93] |
Maize straw and pig manure | Pyrolysis (300 °C and 500 °C) | Distilled water (The pH values of the solution were 1.0, 4.0 and 7.0.) | The relative abundance of humic acid-like materials in BCDOM exceeds 70%. | [94] |
| Rice straw | Pyrolysis (450 ℃, O2-limited) | Milli-Q ultrapure water | BCDOM exhibited high aromaticity, stability, and resistance. Fulvic acid-like and protein-like components were dominant in BCDOM, whereas humic acid-like component was presented at the lowest level. | [95] |
| Dewatered sludge, fresh human manure, and fruit peels | Pyrolysis (200-600 °C) | Ultrapure water | Fresh human manure biochar at lower carbonization temperatures (300-400 ℃) and fruit peel biochar at higher carbonization temperatures (500-600 ℃) have a higher content of aromatic and hydrophobic components. Small molecules in BCDOM were converted to large molecules at 600 ℃. | [50] |
Electrospray ionization coupled to Fourier transform ion cyclotron resonance mass spectrometry (ESI-FTICR-MS) is also frequently utilized to analyze BCDOM components at a molecular level [75, 103, 104]. Based on the analysis of ESI-FTICR-MS, the BCDOM molecules extracted from pinewood biochar were mainly composed of sulfur-containing condensed ligneous components and carbohydrate ligneous components (Figure 3(f)) [105]. In addition, pyrolysis-gas chromatography-mass spectrometry (Py-GC-MS) is considered to be a useful technique to directly investigate BCDOM and provide information on molecular structures [106]. Through Py-GC-MS analysis, the researchers verified that the BCDOM contained nine components, such as lignin products, aldehydes, phenols, methylene chain compounds, polycyclic aromatic hydrocarbons, monocyclic aromatic hydrocarbons, and others [39]. In addition, solid-state 13C nuclear magnetic resonance and liquid chromatography-organic carbon detection (LC-OCD) can explore the morphology of carbon in BCDOM qualitatively and quantitatively [107, 108]. The solid-state 13C nuclear magnetic resonance displayed that the DOC derived from 46 biochar was rich in small fused-ring aromatics, carboxyl C, and aliphatic C [108]. LC-OCD analysis verified that the content of humic acid-like components and bio-polymers in BCDOM extracted from pyrolyzed municipal solid waste biochar at 105 ℃ was higher than that at 450 ℃ [109]. However, current studies focusing on the characterization of BCDOM are incomplete, and further investigation is also warranted for a better understanding of the composition and structure of BCDOM.
4 Interaction of BCDOM with soil organisms
In a complex soil system, there are many coexisting soil organisms such as heavy metals, organic pollutants, soil microorganisms, and plants. The relationship between BCDOM and soil organisms is essential for regulating the destiny of soil organisms and the balance of soil systems. Consequently, this section briefly discusses the effect and mechanism of BCDOM on soil organisms.
4.1 Heavy metals
As a “migration carrier” of heavy metals, biochar can adsorb a large number of heavy metals, such as copper (Cu(II)), lead (Pb(II)), cadmium (Cd(II)), and arsenic (As) by functional groups through adsorption, chemical precipitation, ion exchange, redox reaction, and surface complexation, which would affect the speciation, bioavailability, and distribution of soil heavy metals [110-115]. BCDOM, one of the most active components of biochar, has also been reported to combine with heavy metals by complexation process, reducing their mobility, availability, and toxicity and altering their environmental behavior and fate [116, 117]. Nevertheless, the interaction between BCDOM and heavy metals both has positive and negative effects, i.e., in some cases, the interaction of heavy metals with BCDOM may reduce the adsorption capacity of biochar for heavy metals, thus limiting the effectiveness of biochar in soil remediation. The extent of this interaction is affected by several factors, including the type and concentration of heavy metals, the characteristics of BCDOM, and the properties of soil [53, 111, 118, 119]. Hence, enhanced knowledge and research are required to determine the optimal conditions for utilizing the interaction between BCDOM and heavy metals to control heavy metal pollution in the soil environment. Table 2 shows the impact of BCDOM derived from different biochars on heavy metals.
| BCDOM type | Heavy metals | Performance | Binding functional groups | Reference |
|---|---|---|---|---|
| Corn straw BCDOM | Pb(II) | The adsorption capacity of Pb(II) by corn straw biochar was 10.82 mg/g. | BCDOM was firstly bound to protein-like components and humic acid-like components. | [4] |
| Walnut shells BCDOM | Cu(II) | The maximum removal rate of Cu(II) was 99.8%. | The protein-like materials dominated in terms of the fluorescent components and binding affinity with Cu(II). | [120] |
| Rice straw BCDOM | Cu(II) and Cd(II) | For Cu(II), the binding order was polysaccharide, N=O stretching of aromatic and aliphatic groups, phenolic groups, and carboxyl groups. For Cd(II), the binding process was phenolic and carboxyl groups, polysaccharide and N=O stretching of aromatic groups, aliphatic groups, and C=C stretching of aromatic groups. | [95] | |
| Sludge BCDOM | Cu(II) | In sludge BCDOM, Cu(II) firstly binds to fulvic acid-like and humic acid-like components, and then phenolic hydroxyl-carboxylate, polysaccharide groups, C=C bond of aromatic carbon, aliphatics, and C—O—C bond of aliphatic ethers. | [118] | |
| to be continued | ||||
4.1.1 Copper
Previous studies have manifested that various organic functional groups and fluorophores are recognized as the major sites for BCDOM binding to heavy metals, and affect the bioavailability, mobility, and toxicity of heavy metals through complexation processes [118, 127-129]. For instance, phenolic and thiol function groups in BCDOM have been reported to possess a strong metal-binding ability, and amide functional groups could be involved in Cu(II) binding [119, 122]. For different heavy metals, variations in the composition and content of BCDOM could lead to differences in the order and ability of BCDOM to bind heavy metals [37]. It has been revealed that polysaccharides, aliphatic groups, and aromatic N=O in BCDOM derived from cauliflower root biochar could preferentially react with Cu(II) [126]. From the detailed sequence of Cu(II) binding with BCDOM derived from sludge biochar, WU et al [118] reported that hydrophilic sites bind towards Cu(II) before hydrophobic sites. Additionally, HUANG and coworkers [95] revealed that protein-like and humic acid-like substances possessed stronger binding ability with Cu(II) than fulvic acid-like substances (Figure 4(a)). Similar results were also obtained via the binding process between Cu(II) and BCDOM extracted from sewage sludge biochar [125].
Contined
| BCDOM type | Heavy metals | Performance | Binding functional groups | Reference |
|---|---|---|---|---|
| Human feces BCDOM | Zn(II) and Pb(II) | Pb(II) binding to BCDOM first occurred in protein-like substances, followed by fulvic acid-like substances, and the binding order is COO— stretching in carboxylic acids > the C=O stretching of aromatic > the O—H deformation of phenolic compounds > the C—O stretching of alcohols, polysaccharide, ethers, and esters =C—H bending. | [1] | |
| Fulvic acid-like fractions of BCDOM exhibited a stronger Zn(II) affinity than the protein-like fractions, and the functional groups in BCDOM bind to Zn(II) in the following order: COO— stretching in carboxylic acids > aliphatic C—OH>C—H bending. | ||||
| Straw stalk, rice husk, and cattail BCDOM | Pb(II) | The maximum removal ability of Pb(II) reached 423.9 mg/g. | Fulvic acid-like substances or aliphatic components with low aromaticity in BCDOM were favorable for the formation of BCDOM- Pb(II) complexes. | [121] |
| Raw rapeseed cake BCDOM | Cu(II) | The binding ability of BCDOM to Cu(II) was 2.18-17.7 μmol/L. | Humic acid-like substances showed faster reaction and higher binding efficiency with Cu(II) than that of protein-like substances. | [122] |
| Corn straw BCDOM | Pb(II) | The highest Pb(II) removal efficiency was (62.2±2.5)%. The maximum content of immobilized Pb(II) was 378.1 mg/kg. | Humic acid-like substances could be complex with Pb(II) to promote the separation of Pb(II) from soil colloids. | [4] |
| Wheat straw and corn straw BCDOM | Cd(II) | The protein-like components were more important than humic acid-like components for the interaction between BCDOM and Cd(II). Carboxylic and phenolic groups took part in the binding process of Cd(II) with BCDOM. | [123] | |
| Wetland plants BCDOM | Cu(II) | The quenching ratio of humic acid-like substances ranged from 46% to 94% as the concentration of Cu(II) was increased from 0 to 10 mg/L. | Both humic acid-like and protein-like substances of BCDOM could be complexed with Cu(II). Humic acid-like substances in BCDOM exhibited a higher binding capacity for Cu(II) than other substances. | [124] |
| Rice straw BCDOM | Pb(II) | The binding order of BCDOM with Pb(II) was phenolic hydroxyl groups, aromatic groups, the C=O stretching of amide and carboxylic acid, the C—O stretching of polysaccharides, and the NO2 stretching of aromatic. | [53] | |
| Sewage sludge BCDOM | Cu(II) | Humic acid-like substances possessed high sensitivity to Cu(II) complexation. The binding order of Cu(II) to BCDOM was protein-, humic acid-, and fulvic acid-like fractions. | [125] | |
| Reed straw cauliflower root, potato stalk, and corn stalks BCDOM | Cu(II) and Pb(II) | Polysaccharides and aliphatic groups in four BCDOMs were first combined with Pb(II). The groups of aliphatic, polysaccharides, and aromatic N=O in the four BCDOM exhibited the fastest reaction with Cu(II). | [126] |
Carbonization temperature is known to affect the structure and composition of BCDOM, which may affect the binding order, ability, and mechanism of Cu(II). Generally, lower carbonization temperature was conducive to Cu(II) binding with BCDOM, which was attributed to the decrease of fluorescence substance and aromaticity of BCDOM [125]. For instance, the binding ability of BCDOM extracted from walnut-shell biochar with Cu(II) decreased gradually with increased pyrolysis temperature from 300 to 700 ℃ [120]. By calculating the binding parameters via the Ryan and Weber model, the results implied that fulvic acid-like substances were indispensable in the BCDOM-Cu(II) complexation process at 300 ℃. While at 500 and 700 ℃, Cu(II) preferential bound with protein-like substances. In addition, based on a multi-analytical method, YAN and colleagues [124] reported that tryptophan-like and humic acid-like substances in BCDOM extracted from wetland plants biochar were more sensitive to Cu(II) during pyrolysis at 300 and 500 ℃. However, the binding ability of humic acid-like substances to Cu(II) was more prominent than that of tryptophan-like substances (Figure 4(b)). These results are consistent with previous research [95, 130]. Consequently, BCDOM prepared by low-temperature carbonization was more favorable for binding with Cu(II).
4.1.2 Lead
Pb(II) is considered as a hazardous metal because it is bioaccumulative, non-degradable, and extremely harmful to plant growth and human health [131, 132]. Hence, research is required to investigate the effect of biochar and BCDOM on the distribution, migration, and transformation of Pb(II) in soil. It was found that the ethers, aliphatic, C—O stretching of alcohols, polysaccharides, esters, and phenol hydroxyl groups in different BCDOM could rapidly respond to Pb(II) binding [53, 126, 133]. ZHANG’s group [126] manifested that polysaccharides in BCDOM derived from reed straw biochar gave the first response to Pb(II), and humic acid-like and protein-like substances were the primary substances that participated in the sorption of Pb(II). Another study reported that BCDOM derived from feces biochar pyrolyzed at 280 ℃ processed a higher affinity for Pb(II) compared to pyrolysis at 380 ℃, indicating that humic acid-like and fulvic acid-like fractions of BCDOM displayed a high affinity for Pb(II), and both of them could form stable BCDOM-Pb(II) complexes with Pb(II) [1].
Relevant research displayed that the adsorption of Pb(II) by biochar increased from 5.69 mg/g to 10.82 mg/g as soil DOM was co-adsorbed by biochar, indicating that the presence of DOM could promote the adsorption of heavy metals by biochar [4]. Therefore, to further elucidate the contribution of BCDOM to the adsorption of Pb(II) on biochar, a series of experiments for adsorption of Pb(II) by BCDOM extracted from cattail, straw stalk, and rice husk biochar were carried out [121]. Results displayed that the total adsorbed quantity of Pb(II) on bulk biochar was higher than that of residual biochar without BCDOM fraction, manifesting that BCDOM promoted the adsorption of Pb(II) by biochar due to the complexation between Pb(II) and BCDOM. More importantly, the contribution of BCDOM for Pb(II) adsorption mainly involved van der Waals at low pyrolysis temperatures, whereas the ligand exchange mechanism played a key role at high pyrolysis temperatures. In addition, according to the leaching tests, LIU et al [60] found that the concentration of Pb(II) decreased from (295.5± 3.7) mg/kg to (20.5±2.5) mg/kg after extracting corn straw BCDOM from red soil, where about 93% of Pb(II) was removed, suggesting that the BCDOM possessed great ability to bond with Pb(II). Hence, the above findings manifest that the existence of BCDOM can enhance the adsorption efficiency of Pb(II) on biochar.
4.1.3 Cadmium
Cd(II) is considered to be a nephrotoxicant that can cause a variety of pathological, such as renal dysfunction, high blood pressure, and cancers [3]. Therefore, the treatment of Cd(II) pollution is urgent and significant. One study reported that DOM extracted from microbe promoted the adsorption of Cd(II) in soil and decreased Cd(II) migration and bioavailability to plants, manifesting that DOM was conductive to the control and treatment of Cd(II) pollution [36]. However, after the removal of BCDOM from biochar produced by pyrolysis of wheat straw at low temperatures, the residual biochar exhibited a better immobilization of heavy metals (Cd(II), Pb(II), Cu(II) and Zn(II)) in soil, and the immobilization rate could reach 27%-78% [46]. The reason was that the residual biochar after BCDOM removal possessed abundant functional groups and porous structure, promoting the adsorption of heavy metals on biochar [113]. However, the removal efficiency of Cd(II) by BCDOM as well as the interaction mechanism are unclear, and further studies are required.
In general, the interaction between BCDOM with low molecular hydrophilic and heavy metals was conducive to the dissolution and transportation of soil heavy metals, reducing their bioavailability and solubility via complexation, adsorption, and chelation [108, 134-136]. To investigate the interaction between BCDOM and Cd(II), a series of quenching titration experiments were performed [95]. Results displayed that protein-like and fulvic acid-like components in BCDOM extracted from rice straw biochar processed stronger binding ability for Cd(II). Moreover, two-dimension correlation spectroscopy (2D-COS) analysis indicated that carboxyl and phenolic groups in BCDOM were dominant in binding to Cd(II) via the complexation process. In addition, GONG and coworkers [137] found that the total adsorption capacity of Cd(II) by magnetic straw biochar was increased by 3.6-fold after removing BCDOM from biochar, which was mainly attributed to the exposure of more adsorption sites, leading to the enhancement of Cd(II) adsorption by ion exchange and precipitation. Therefore, the results of these studies proved that the presence of BCDOM might promote migration but hinder the adsorption capacity of Cd(II) by biochar.
4.1.4 Arsenic
As is a highly toxic trace element that can accumulate in the body through the food chain and water, leading to diseases such as lung and skin cancer [138, 139]. The International Agency for Research on Cancer already considers inorganic As to be a highly toxic carcinogen [140]. The influence of biochar-modified soil on the mobility and availability of As has been investigated in recent years. It has been reported that the bioaccumulation of As in rice plants was reduced by 88% after the addition of sewage sludge biochar [127]. Another study found that the As mobility decreased with decreasing the amount of DOC released from sludge, coffee waste, and rice straw biochar, suggesting that BCDOM plays a pivotal role in the transportation of As in As-contaminated soils (Figure 4(c)) [141]. Incubation experiments revealed a significant increase in acid-soluble As after the application of rice straw biochar in soil, which was ascribed to the fact that the addition of biochar increased the concentration of DOM in the soil, resulting in an increase of extractable As [142]. Additionally, KIM’s team [57] discovered that the mobility of As was minimized when lignin-rich biochar was applied to the As-contaminated soil, which was attributed to the content of BCDOM in biochar decreased with the increment of lignin content. Therefore, controlling the lignin content of biochar can further enhance the removal capacity of biochar to As. However, the mechanism of biochar and BCDOM enhanced the mobility of As had not been identified exactly, which is worthy of in-depth research.
4.2 Organic pollutants
According to previous studies, biochar could increase the adsorption of organic compounds and decrease the mobility and availability of organic pollutants in the soil, altering the transport and fate of these contaminants [59, 94, 120, 143]. As one of the most active components of biochar, BCDOM can participate in the complexation of soil organic pollutants directly or indirectly, influencing the migration and transformation of soil organic pollutants. Similar to heavy metals, the interaction efficiency and mechanism between BCDOM and organic pollutants are also affected by the nature and concentration of organic pollutants, the properties of BCDOM, and the characteristics of soil [92, 144, 145]. For instance, TANG et al [56] reported that BCDOM extracted from soybean straw and rice straw biochar displayed higher adsorption capacity for polycyclic aromatic hydrocarbon compounds than those derived from sewage sludge, peanut shells, and mushroom waste biochar. It has been reported that the adsorption efficiency of phenol by biochar increased from 63.3% to 70.3% when the concentration of DOM added was increased from 0 to 1000 mg/L [146]. Additionally, the existence of BCDOM derived from rice straw biochar could shorten the adsorption equilibrium time of sulfamethoxazole and chloramphenicol, and increase the adsorption efficiency of sulfamethoxazole on biochar [147]. The reason for this phenomenon was that BCDOM might act as a biochar-like adsorbent to adsorb sulfamethoxazole via electron exchange [148, 149].
However, some studies exhibit the inverse trends. It has been reported that the combination of organic pollutants with BCDOM may reduce the efficiency of biochar in soil remediation by diminishing its capacity to adsorb organic pollutants [135, 145]. For instance, BCDOM derived from corn straw biochar could seriously suppress (66.0%) the adsorption of bisphenol S on corn straw biochar [92]. Another study found that BCDOM severely inhibited the adsorption of chloramphenicol on rice straw biochar, probably due to the competitive adsorption of BCDOM with chloramphenicol to the biochar adsorption sites [147]. DENG et al [144] further reported that the Kf value, represented as an indicator of the adsorption capacity of biochar to bisphenol A, increased from 450.16 to 737.37 after removing the BCDOM from corn straw biochar, which could be attributed to the dissolution of BCDOM releasing the blocked pores on the biochar surface. Moreover, the results of 2D-COS analysis further indicated that the affinity quenching effect between protein-like substances of BCDOM and bisphenol A was stronger than that of humic acid-like substances (Figure 5(a)). The contrary results from different studies indicated that BCDOM could simultaneously play a two-way roles in the removal of pollutant: on the first hand, BCDOM might participate in the pollutants degradation and promote the removal of pollutants; On the other hand, BCDOM might block the pores on the surface of biochar, preventing the adsorption of pollutants. Therefore, more research is necessary to enhance the utilization of the interaction between organic pollutants and BCDOM to alleviate organic pollutants in soils.
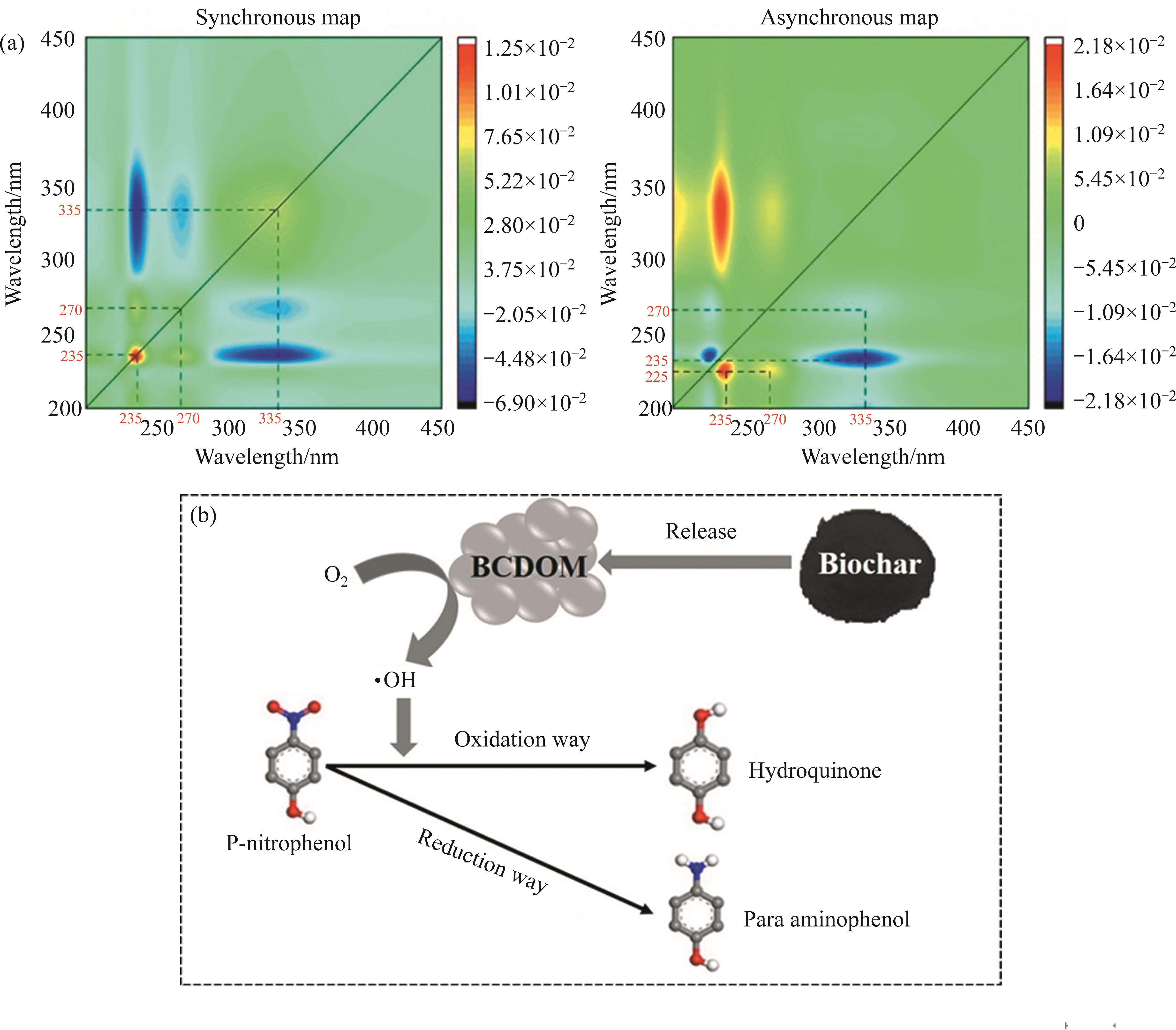
To date, although the adsorption mechanism of organic contaminants by biochar has been explored, the interaction mechanism between BCDOM and organic pollutants remains unclear. Therefore, it is of great significance to research the interaction mechanism between organic pollutants and BCDOM. Numerous studies have reported that BCDOM is combined with organic pollutants through various mechanisms, including aromatic stacking, electrostatic interaction, hydrogen bond, and hydrophobic force [150-153]. It has been reported that BCDOM can combined with phenol through π-π interactions and hydrogen bonding, which significantly improves the adsorption of phenol on biochar [146]. In addition, BCDOM could act as an electron donor to generate reactive free radicals, such as hydroxyl, alkoxy, and sulfate radicals, for the degradation of organic pollutants through redox reactions [154, 155]. ZHANG’s group [91] reported that the removal of p-nitrophenol by BCDOM extracted from pine wood biochar mainly involved the formation of complexes between BCDOM and p-nitrophenol, and the degradation of p-nitrophenol by BCDOM through oxidative and reductive processes. The degradation extent of p-nitrophenol by BCDOM reached 9.54 mg/mg, which was hundreds of times higher than that of biochar (0.0099-0.012 mg/mg), indicating that BCDOM possessed a much higher degradation ability for p-nitrophenol than biochar (Figure 5(b)). As a result, it could be concluded that the performance and mechanism of BCDOM removing organic pollutants were closely associated with the types and properties of BCDOM and organic pollutants. However, the primary influencing factors and the exact mechanism of organic contaminant degradation by BCDOM should be further investigated to accurately assess and manipulate the environmental effects of biochar application.
4.3 Soil microorganisms
In the whole soil ecosystem, soil microorganisms are considered to be the core components of soil due to their ability to remediate soil properties through decomposition, bioremediation, soil aggregate formation, soil contaminants stabilization, and nutrient cycling [156]. Therefore, soil microbial communities are commonly used as indicators of the extent of biochar remediation and soil quality [157]. It has been reported that the microbial community and activity might potentially be influenced by the components of biochar, such as BCDOM, volatile organic compounds, minerals, and free radicals [158]. Under normal circumstances, microorganisms can feed on BCDOM, and the plentiful porous structure of biochar will offer carriers and habitats for microorganisms, indicating that BCDOM can affect the microbial growth or microbial communities [43, 159-161]. For instance, YANG et al [7] revealed that the soil microbial activity was strongly related to BCDOM components, especially fulvic acid-like and tryptophan-like components. Another study found that the structure of microbial communities was greatly varied after incubation with BCDOM extracted from maize straw and pig manure biochar [94]. The results displayed that the content of aromatic substances in BCDOM was positively correlated with the relative abundances of Methylotenera, Acinetobacter, and Reyranella. Whereas, the relative abundances of Dyadobacter, Novosphingobium, and Sphingobacterium were positively correlated with the content of aliphatic compounds in BCDOM. However, different from the influence of BCDOM on soil heavy metals and organic pollutants, the impacts of BCDOM on soil microbial activity are probably more complex, and the mechanism of BCDOM stimulating microorganisms is unclear.
4.4 Soil plants
Biochar, as a soil amendment, has attracted wide consideration due to its excellent physicochemical properties [162, 163]. Similarly, BCDOM with a variety of active groups has been indicated to play a role in buffering soil acidity and alkalinity, creating a favorable soil environment for plant growth [12, 30, 36]. In addition, BCDOM could be migrated from soil to surface water and groundwater via leaching and surface runoff, resulting in the migration of some contaminants bound to BCDOM, thereby altering the physicochemical and biological properties of soil [164]. As a nutrient, BCDOM can also provide plants with humic components, neutral compounds, low-molecular-weight acids, some macro- and micro-nutrient elements, and other materials, which has been demonstrated to be beneficial for seed germination and plant growth [44, 45, 165]. One study reported that the plant metabolic processes were significantly promoted by labile organic molecules released from rice husks [165]. By application of BCDOM solution derived from maize and wheat biochar, the nutrition quality and yield of Chinese cabbage were significantly promoted [166]. The shoot biomass and leaf-soluble sugar content increased by 89% and 83%, respectively. Particularly, BCDOM could increase the gene expression levels of glutamine synthetase enzyme activity and nitrate reductase in the test plants [46]. Previous studies also illustrated that BCDOM could enhance the resistance of plants to generalized mites, Leveillula taurica, and leaf fungal pathogens Botrytis cinerea, which leads to increasing resistance to plants and promoting plants’ growth [167, 168]. All of these results suggested that the presence of BCDOM could promote the growth of plants in soil, especially in biochar-remediated soil.
5 Transport of BCDOM in soil environmental
In general, the main mechanism of soil-derived DOM (SDOM) retention in soil is adsorption by soil solids during migration [169, 170]. By evaluating the transport process of SDOM in soil, it was found that the retention of SDOM in forest soil was stronger than that in podzolic soil or high clay-content soil [171]. Especially, aromatic SDOM moieties were preferentially retained during the transport process of SDOM in soil [172]. Additionally, through the osmosis process, SDOM could transfer carbon and nutrients with soil solution to deeper layers, indicating that SDOM could be stored or transported from the terrestrial to aquatic system [32, 173]. However, it is still unclear whether the transport process of BCDOM in the soil is similar to that of SDOM. Therefore, more attention should be paid to the transformation and migration process of BCDOM in soil.
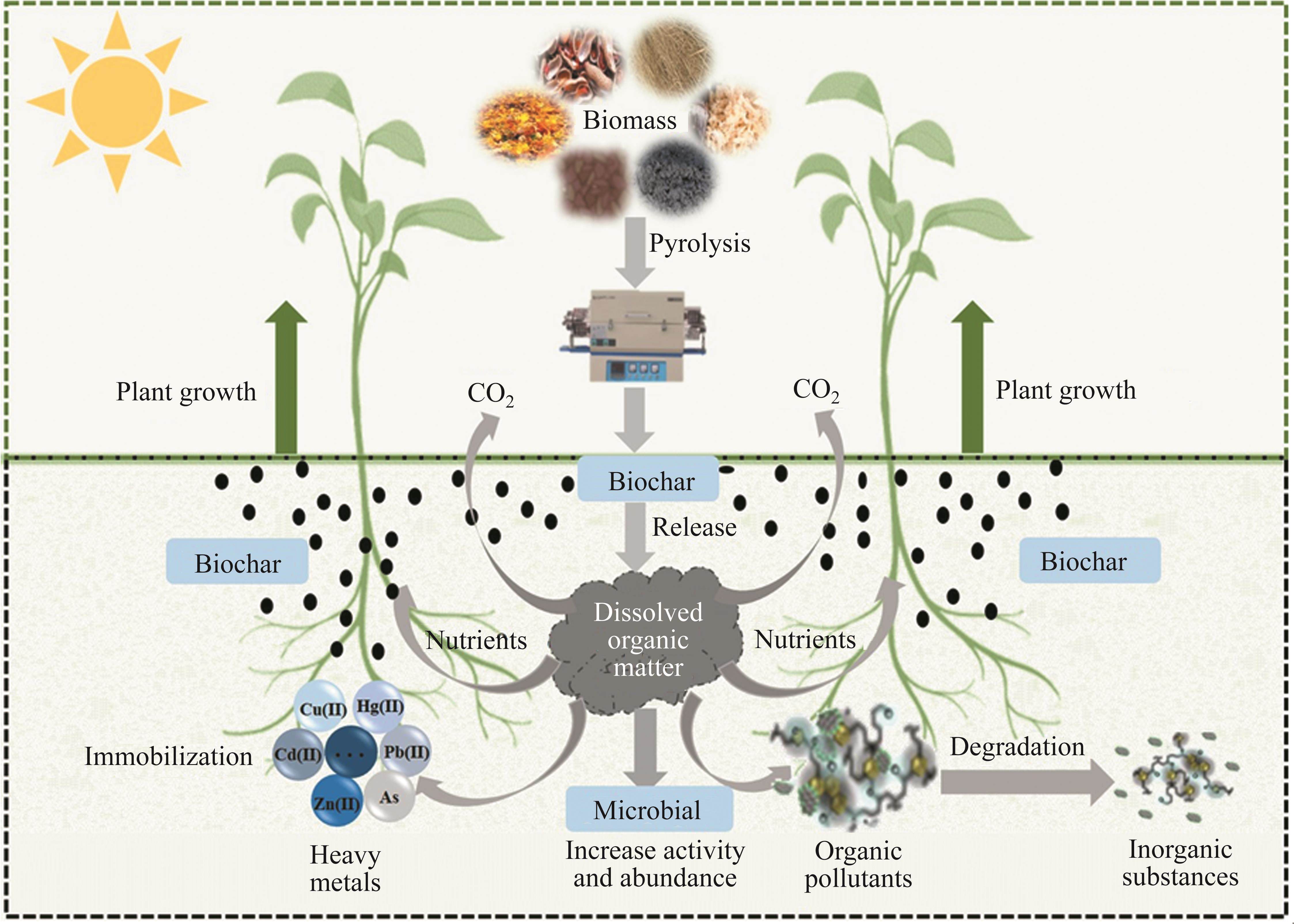
Generally, the transportation and decomposition of BCDOM in soil are correlated with the environment of soil, such as surface runoff, soil structure, soil microbial activity, and hydraulic properties [108, 174-176]. To investigate the decomposition and transportation process of BCDOM, a series of microbial incubation experiments were conducted [95]. As the incubation period lengthened, the values of SUVA254 increased gradually, indicating that the simple and unstable matrix in BCDOM was transformed into an aromatic carbon structure by microorganisms [175]. After 14 d of incubation, the maximum fluorescence intensity of protein-like substances declined while the values of humic acid-like substances displayed an increasing trend, manifesting that a proportion of protein-like substances in BCDOM was transformed into humic acid-like substances that could be stabilized in the soil. Additionally, through the experiments of biologically aged biochar, QUAN et al [177] further manifested that aliphatic and non-aromatic portions were the labile portion of BCDOM, which could be easily lost via microbial mineralization. Therefore, the transport and transformation processes of BCDOM in the soil system and its impact on the environmental system can be represented in Figure 6. The biological activity of BCDOM can stimulate the activity and abundance of microorganisms, fix heavy metals, and degrade organic pollutants. In contrast, under the action of microorganisms, BCDOM may undergo mineralization. Some of the products provide nutrients for plant growth and are eventually converted into small CO2 molecules that are released into the environment. However, the specific process and mechanism of microorganism action on the migration and transformation of BCDOM in soil still needs further research.
6 Potential risks of BCDOM
BCDOM has received increasing attention in recent years as a factor influencing soil contaminants, microbial abundance and community structure, and even soil environmental systems. However, some negative effects may also be introduced into the soil environment by BCDOM. For instance, due to the presence of potentially toxic organic matters in BCDOM, such as polycyclic aromatic hydrocarbons, phenol, heterocyclic nitrogen, and furans, toxic effects might be introduced during the application of biochar and BCDOM [178, 179]. SMITH et al [48] found that the BCDOM extracted from pinewood biochar was toxic to eukaryotic green alga (Desmodesmus) and blue-green alga (cyanobacteria Synechococcus), which extremely inhibited the growth of aquatic photosynthetic microorganisms. Additionally, soil microorganisms might also be affected by the toxicity of BCDOM [158]. HAO et al [180] reported that when the pyrolyzed temperature increased to 330 ℃, the biotoxicity of BCDOM derived from hydrochar increased dramatically and was significantly higher than that of low production temperature, which was ascribed to the enhancement of organic acids, phenols, and other organic compounds containing oxygen groups. Bioassay results displayed that the cyanobacterial growth was completely suppressed by the hydrochar-DOM produced at 330 ℃. Therefore, to avoid the toxic effects of biochar and BCDOM, the selection of suitable feedstocks and preparation processes is necessary. Additionally, BCDOM may compete with contaminants in soil for adsorption sites, leading to limited adsorption efficiency of biochar. YANG and colleagues [92] found that the adsorption capacity of biochar to bisphenol S was suppressed because of competing adsorption between BCDOM and bisphenol S. On the biochar surface, BCDOM competed with perfluorinated and polyfluorinated substance substances for adsorption sites, which reduced the removal rate of perfluorinated and polyfluorinated substance [145]. In addition to the adsorption of pollutants, the competitive adsorption between BCDOM and nutrients in the soil might also occur. It has been reported that the migration of phosphate in the soil was increased by competitive adsorption of BCDOM and phosphate [181]. Hence, considering the potential negative effects of BCDOM, special attention needs to be paid to an in-depth understanding of the negative effects of BCDOM.
7 Conclusions and future perspective
The utilization of biochar in soil has great advantages for the rapid and healthy development of sustainable agricultural systems. BCDOM, as a small but important component of biochar, plays an important role in determining the soil remediation performance of biochar. The presence of BCDOM may both have positive or negative effects on soil contaminants as well as the soil environment. The quantity of BCDOM increases with the decrease in carbonization temperature and the lignin content of biochar feedstock. Moreover, the increase of the basicity of the extract solution was also beneficial to the release of BCDOM from biochar. In the soil environment, BCDOM interacts with heavy metals through adsorption and complexation, thereby promoting the immobilization of heavy metals and reducing their mobility and toxicity. More importantly, protein-like substances in BCDOM were the primarily active substances that bind to heavy metals, including Cu(II), Pb(II), and Cd(II). BCDOM can enhance the degradation of organic pollutants by biochar through complexation and redox processes, and demonstrate excellent performance in improving microbial activity, soil nutrients, and soil properties for plant growth. However, there are some potential risks of BCDOM to soil environment. Hence, special attention should be given to the selection of the preparation method of biochar and extraction method of BCODM, and the mechanism of BCDOM and soil pollutants in future research.
Considering the research and application of BCDOM, future research directions on BCDOM should focus on the following: 1) On account of the aforementioned analysis, the content, composition, and properties of BCDOM depend on the biochar preparation. Thus, to enhance the performance of BCDOM and biochar, it is crucial to control the composition and content of BCDOM by optimizing the biochar feedstock and preparation process; 2) Extraction methods have significant influence on the composition and properties of BCDOM. However, there are no standard methods for extracting the BCDOM from biochar. Therefore, more attention should be given to the extraction methods of BCDOM to obtain a clearer understanding of the composition of BCDOM and its impact on environmental systems; 3) Advanced characterization technologies are also urgently needed for a deeper understanding and utilization of BCDOM; 4) The transport and transformation processes of BCDOM in the soil system and its effects on the soil contaminants, microorganisms, and plant growth in soil ecosystems during biochar application are not fully understood. Hence, further research in this area should be conducted to precisely assess and manipulate the ecological effects of BCDOM and biochar applications, improving the efficiency of biochar remediation of soils. 5) To further enhance the application of biochar, more consideration should be given to the potential environmental risks and long-term ecological influences of BCDOM in the soil environment as well as the whole ecosystem. Overall, this review will be conducive to the development and practical application of biochar and BCDOM in soil remediation.
Binding characteristics of Pb and Zn to low-temperature feces-based biochar-derived DOM revealed by EEM-PARAFAC combined with general and moving-window two-dimensional correlation analyses
[J]. Environmental Science and Pollution Research International, 2023, 30(10): 27525-27538. DOI: 10.1007/s11356-022-24132-z.Pyrolysis temperature-dependent changes in the characteristics of biochar-borne dissolved organic matter and its copper binding properties
[J]. Bulletin of Environmental Contamination and Toxicology, 2019, 103(1): 169-174. DOI: 10.1007/s00128-018-2392-7.Health risk assessment of dietary cadmium intake: Do current guidelines indicate how much is safe?
[J]. Environmental Health Perspectives, 2017, 125(3): 284-288. DOI: 10.1289/EHP108.Sorption of Pb(II) onto biochar is enhanced through co-sorption of dissolved organic matter
[J]. Science of the Total Environment, 2022, 825: 153686. DOI: 10.1016/j.scitotenv. 2022.153686.Mechanical activation of natural chalcopyrite for improving heterogeneous Fenton degradation of tetracycline
[J]. Journal of Central South University, 2022, 29(12): 3884-3895. DOI: 10.1007/s11771-022-5199-y.Phosphorus adsorption by functionalized biochar: A review
[J]. Environmental Chemistry Letters, 2023, 21(1): 497-524. DOI: 10.1007/s10311-022-01519-5.Effect of gasification biochar application on soil quality: Trace metal behavior, microbial community, and soil dissolved organic matter
[J]. Journal of Hazardous Materials, 2019, 365: 684-694. DOI: 10.1016/j.jhazmat.2018.11.042.Biochar-based slow-release of fertilizers for sustainable agriculture: A mini review
[J]. Environmental Science and Ecotechnology, 2022, 10: 100167. DOI: 10.1016/j.ese.2022.100167.Advances in in situ and ex situ tar reforming with biochar catalysts for clean energy production
[J]. Sustainable Energy and Fuels, 2018, 2(2): 326-344. DOI: 10.1039/C7SE00553A.Improving water electrolysis assisted by anodic biochar oxidation for clean hydrogen production
[J]. Energy, 2022, 238: 121793. DOI: 10.1016/j.energy.2021.121793.Role of tobacco and bamboo biochar on food waste digestate co-composting: Nitrogen conservation, greenhouse gas emissions, and compost quality
[J]. Waste Management, 2023, 156: 44-54. DOI: 10.1016/j.wasman.2022.10.022.Biochar properties and eco-friendly applications for climate change mitigation, waste management, and wastewater treatment: A review
[J]. Renewable and Sustainable Energy Reviews, 2017, 79: 255-273. DOI: 10.1016/j.rser.2017.05.057.Roles of biochar-derived dissolved organic matter in soil amendment and environmental remediation: A critical review
[J]. Chemical Engineering Journal, 2021, 424: 130387. DOI: 10.1016/j.cej.2021.130387.A review on persulfates activation by functional biochar for organic contaminants removal: Synthesis, characterizations, radical determination, and mechanism
[J]. Journal of Environmental Chemical Engineering, 2021, 9(5): 106267. DOI: 10.1016/j.jece.2021.106267.Research advances on production and application of algal biochar in environmental remediation
[J]. Environmental Pollution, 2024, 348: 123860. DOI: 10.1016/j.envpol.2024. 123860.How does ball-milling elevate biochar as a value-added peroxydisulfate activator for antibiotics removal?
[J]. Industrial Crops and Products, 2024, 214: 118569. DOI: 10. 1016/j.indcrop.2024.118569.Highly dispersed iron-doped biochar derived from sawdust for Fenton-like degradation of toxic dyes
[J]. Journal of Cleaner Production, 2021, 297: 126681. DOI: 10.1016/j.jclepro. 2021.126681.Low tech biochar production could be a highly effective nature-based solution for climate change mitigation in the developing world
[J]. Plant and Soil, 2022, 479(1): 77-83. DOI: 10.1007/s11104-021-05159-6.Biochar in climate change mitigation
[J]. Nature Geoscience, 2021, 14: 883-892. DOI: 10.1038/s41561-021-00852-8.Biochar produced from wood waste for soil remediation in Sweden: Carbon sequestration and other environmental impacts
[J]. Science of the Total Environment, 2021, 776: 145953. DOI: 10.1016/j.scitotenv.2021.145953.A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis
[J]. Agriculture, Ecosystems and Environment, 2011, 144(1): 175-187. DOI: 10.1016/j.agee.2011.08.015.Dissolved phosphorus speciation of flash carbonization, slow pyrolysis, and fast pyrolysis biochars
[J]. ACS Sustainable Chemistry and Engineering, 2015, 3(7): 1642-1649. DOI: 10.1021/acssuschemeng.5b00336.Role of biochar on composting of organic wastes and remediation of contaminated soils-a review
[J]. Environmental Science and Pollution Research International, 2017, 24(20): 16560-16577. DOI: 10.1007/s11356-017-9168-1.Role of biochar-derived DOM compositions in enhanced biodegradation of sulfamethoxazole and chloramphenicol
[J]. Journal of Hazardous Materials, 2023, 458: 131979. DOI: 10.1016/j.jhazmat.2023.131979.Remediation of heavy metal contaminated soils by biochar: Mechanisms, potential risks and applications in China
[J]. Environmental Pollution, 2019, 252: 846-855. DOI: 10.1016/j.envpol.2019.05.151.Highly effective stabilization of Cd and Cu in two different soils and improvement of soil properties by multiple-modified biochar
[J]. Ecotoxicology and Environmental Safety, 2021, 207: 111294. DOI: 10.1016/j.ecoenv.2020.111294.The crucial factors of soil fertility and rapeseed yield - A five year field trial with biochar addition in upland red soil, China
[J]. Science of the Total Environment, 2019, 649: 1467-1480. DOI: 10.1016/j.scitotenv.2018.08.412.Biochar effect on water evaporation and hydraulic conductivity in sandy soil
[J]. Pedosphere, 2016, 26(2): 265-272. DOI: 10.1016/S1002-0160(15)60041-8.Production of biochar from renewable resources
[M]//Advanced Technology for the Conversion of Waste into Fuels and Chemicals.Preparation and modification of biochar materials and their application in soil remediation
[J]. Applied Sciences, 2019, 9(7): 1365. DOI: 10.3390/app9071365.Effect of pyrolysis temperature on the composition of DOM in manure-derived biochar
[J]. Ecotoxicology and Environmental Safety, 2020, 197: 110597. DOI: 10.1016/j.ecoenv.2020.110597.Processes that influence dissolved organic matter in the soil: A review
[J]. Scientia Agricola, 2020, 77(3):Water extractable organic carbon in untreated and chemical treated biochars
[J]. Chemosphere, 2012, 87(2): 151-157. DOI: 10.1016/j.chemosphere.2011.12.007.Water extract from straw biochar used for plant growth promotion: An initial test
[J]. BioResources, 2015, 11(1): 249-266. DOI: 10.15376/biores.11.1.249-266.Chemical characterization of dissolved organic matter (DOM): A prerequisite for understanding UV-induced changes of DOM absorption properties and bioavailability
[J]. Aquatic Sciences, 2009, 71(2): 104-126. DOI: 10.1007/s00027-008-8082-5.Effects of exogenous dissolved organic matter on the adsorption-desorption behaviors and bioavailabilities of Cd and Hg in a plant-soil system
[J]. Science of the Total Environment, 2020, 728: 138252. DOI: 10.1016/j.scitotenv.2020.138252.Chemical and structural properties of dissolved black carbon released from biochars
[J]. Carbon, 2016, 96: 759-767. DOI: 10.1016/j.carbon.2015.09.106.The comparison of dissolved organic matter in hydrochars and biochars from pig manure
[J]. Science of the Total Environment, 2020, 720: 137423. DOI: 10.1016/j.scitotenv. 2020.137423.Effect of biochar amendment on organic matter and dissolved organic matter composition of agricultural soils from a two-year field experiment
[J]. Science of the Total Environment, 2022, 812: 151422. DOI: 10.1016/j.scitotenv.2021.151422.Biochar amendment to soil changes dissolved organic matter content and composition
[J]. Chemosphere, 2016, 142: 100-105. DOI: 10.1016/j.chemosphere.2015.04.087.Biochar affects methylmercury production and bioaccumulation in paddy soils: Insights from soil-derived dissolved organic matter
[J]. Journal of Environmental Sciences, 2022, 119: 68-77. DOI: 10.1016/j.jes.2022.02.011.Change in active microbial community structure, abundance and carbon cycling in an acid rice paddy soil with the addition of biochar
[J]. European Journal of Soil Science, 2016, 67(6): 857-867. DOI: 10.1111/ejss.12388.Biochars change the sorption and degradation of thiacloprid in soil: Insights into chemical and biological mechanisms
[J]. Environmental Pollution, 2018, 236: 158-167. DOI: 10.1016/j.envpol.2018.01.030.Effect of clay and iron sulphate on volatile and water-extractable organic compounds in bamboo biochars
[J]. Journal of Analytical and Applied Pyrolysis, 2018, 133: 22-29. DOI: 10.1016/j.jaap.2018.05.007.Chemical aging changed aggregation kinetics and transport of biochar colloids
[J]. Environmental Science and Technology, 2019, 53(14): 8136-8146. DOI: 10.1021/acs.est.9b00583.Biochar DOM for plant promotion but not residual biochar for metal immobilization depended on pyrolysis temperature
[J]. Science of the Total Environment, 2019, 662: 571-580. DOI: 10.1016/j.scitotenv.2019.01.224.Impact of biochar-induced vertical mobilization of dissolved organic matter, sulfamethazine and antibiotic resistance genes variation in a soil-plant system
[J]. Journal of Hazardous Materials, 2021, 417: 126022. DOI: 10.1016/j.jhazmat.2021. 126022.Investigation into the sources of biochar water-soluble organic compounds and their potential toxicity on aquatic microorganisms
[J]. ACS Sustainable Chemistry and Engineering, 2016, 4(5): 2550-2558. DOI: 10.1021/acssuschemeng.5b01687.Pentacyclic triterpenes with selective bioactivity from sebastiania Adenophora leaves, Euphorbiaceae
[J]. Journal of Chemical Ecology, 2007, 33(1): 147-156. DOI: 10.1007/s10886-006-9208-7.The spectral characteristics of biochar-derived dissolved organic matter at different pyrolysis temperatures
[J]. Journal of Environmental Chemical Engineering, 2021, 9(5): 106075. DOI: 10.1016/j.jece.2021.106075.Dissolved organic matter characterization of biochars produced from different feedstock materials
[J]. Journal of Environmental Management, 2019, 233: 393-399. DOI: 10.1016/j.jenvman.2018.12.069.Characteristics of dissolved organic matter composition in biochar: Effects of feedstocks and pyrolysis temperatures
[J]. Environmental Science and Pollution Research International, 2023, 30(36): 85139-85153. DOI: 10.1007/s11356-023-28431-x.Dissolved organic matter released from rice straw and straw biochar: Contrasting molecular composition and lead binding behaviors
[J]. Science of the Total Environment, 2020, 739: 140378. DOI: 10.1016/j.scitotenv.2020.140378.Biochar-derived dissolved organic matter (BDOM) and its influence on soil microbial community composition, function, and activity: A review
[J]. Critical Reviews in Environmental Science and Technology, 2023, 53(21): 1912-1934. DOI: 10.1080/10643389.2023.2190333.Predicting potential release of dissolved organic matter from biochars derived from agricultural residues using fluorescence and ultraviolet absorbance
[J]. Journal of Hazardous Materials, 2017, 334: 86-92. DOI: 10.1016/j.jhazmat.2017.03.064.Spectroscopic characterization of dissolved organic matter derived from different biochars and their polycylic aromatic hydrocarbons (PAHs) binding affinity
[J]. Chemosphere, 2016, 152: 399-406. DOI: 10.1016/j.chemosphere.2016.03.016.Mobility of arsenic in soil amended with biochar derived from biomass with different lignin contents: Relationships between lignin content and dissolved organic matter leaching
[J]. Chemical Engineering Journal, 2020, 393: 124687. DOI: 10.1016/j.cej.2020.124687.In-depth comparison of the physicochemical characteristics of bio-char derived from biomass pseudo components: Hemicellulose, cellulose, and lignin
[J]. Journal of Analytical and Applied Pyrolysis, 2019, 140: 195-204. DOI: 10.1016/j.jaap.2019.03.015.Characteristics and chlorine reactivity of biochar-derived dissolved organic matter: Effects of feedstock type and pyrolysis temperature
[J]. Water Research, 2022, 211: 118044. DOI: 10.1016/j.watres.2022.118044.Characterization of dissolved organic matter in biochar derived from various macroalgae (phaeophyta, rhodophyta, and chlorophyta): Effects of pyrolysis temperature and extraction solution pH
[J]. Science of the Total Environment, 2023, 869: 161786. DOI: 10.1016/j.scitotenv.2023.161786.A bibliometric analysis of biochar application in wastewater treatment from 2000 to 2021
[J]. International Journal of Environmental Science and Technology, 2023, 20(12): 13957-13974. DOI: 10.1007/s13762-023-05030-4.Insights into biochar and hydrochar production and applications: A review
[J]. Energy, 2019, 171: 581-598. DOI: 10.1016/j.energy.2019.01.035.Rapid conversion of red mud into soil matrix by co-hydrothermal carbonization with biomass wastes
[J]. Journal of Environmental Chemical Engineering, 2021, 9(5): 106039. DOI: 10.1016/j.jece.2021.106039.Toxicity of biochars after polycyclic aromatic hydrocarbons removal by thermal treatment
[J]. Ecological Engineering, 2015, 75: 79-85. DOI: 10.1016/j.ecoleng.2014.11.004.Biochar as simultaneous shelter, adsorbent, pH buffer, and substrate of Pseudomonas citronellolis to promote biodegradation of high concentrations of phenol in wastewater
[J]. Water Research, 2020, 172: 115494. DOI: 10.1016/j.watres.2020.115494.A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications
[J]. Renewable and Sustainable Energy Reviews, 2015, 45: 359-378. DOI: 10.1016/j.rser. 2015.01.050.Hydrothermal carbonization of lipid extracted algae for hydrochar production and feasibility of using hydrochar as a solid fuel
[J]. Energy, 2018, 153: 913-920. DOI: 10.1016/j.energy.2018.04.112.Hydrothermal treatment of alkaline red mud and sewage sludge: Formation of a soil-like matrix
[J]. Environmental Technology, 2024, 45(10): 2012-2021. DOI: 10.1080/09593330.2022.2162442.Biochars and hydrochars prepared by pyrolysis and hydrothermal carbonisation of pig manure
[J]. Waste Management, 2018, 79: 395-403. DOI: 10.1016/j.wasman. 2018.08.015.The effect of biochar, hydrochar particles and dissolved organic matter on the photodegradation of metribuzin herbicide in aquatic media
[J]. Journal of Environmental Chemical Engineering, 2021, 9(1): 105027. DOI: 10.1016/j.jece.2021.105027.Molecular and microbial insights towards understanding the effects of hydrochar on methane emission from paddy soil
[J]. Science of the Total Environment, 2020, 714: 136769. DOI: 10.1016/j.scitotenv.2020.136769.Revealing the impact of pyrolysis temperature on dissolved organic matter released from the biochar prepared from Typha orientalis
[J]. Chemosphere, 2019, 228: 264-270. DOI: 10.1016/j.chemosphere.2019.04.143.Influence of thermal air oxidation on the chemical composition and uranium binding property of intrinsic dissolved organic matter from biochar
[J]. Chemosphere, 2023, 317: 137896. DOI: 10.1016/j.chemosphere.2023. 137896.Characterization of dissolved organic matter released from aged biochar: A comparative study of two feedstocks and multiple aging approaches
[J]. Molecules, 2023, 28(11): 4558. DOI: 10. 3390/molecules28114558.Aqueous leaching of organic acids and dissolved organic carbon from various biochars prepared at different temperatures
[J]. Journal of Environmental Quality, 2015, 44(2): 684-695. DOI: 10.2134/jeq2014.08.0341.Organic carbon and nutrient release from a range of laboratory-produced biochars and biochar–soil mixtures
[J]. Geoderma, 2013, 193: 122-130. DOI: 10.1016/j.geoderma.2012.10.002.Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar
[J]. Bioresource Technology, 2012, 107: 419-428. DOI: 10.1016/j.biortech. 2011.11.084.Slow pyrolysis of different Brazilian waste biomasses as sources of soil conditioners and energy, and for environmental protection
[J]. Journal of Analytical and Applied Pyrolysis, 2015, 113: 434-443. DOI: 10.1016/j.jaap.2015.03.006.Effects of pyrolysis temperature and residence time on physicochemical properties of different biochar types
[J]. Acta Agriculturae Scandinavica, Section B - Soil and Plant Science, 2017, 67(1): 12-22. DOI: 10.1080/09064710.2016.1214745.Pyrolysis temperature-dependent release of dissolved organic carbon from plant, manure, and biorefinery wastes
[J]. Journal of Analytical and Applied Pyrolysis, 2013, 104: 84-94. DOI: 10.1016/j.jaap.2013.09.003.Characterization of fluorescent dissolved organic matter from green macroalgae (Ulva prolifera)-derived biochar by excitation-emission matrix combined with parallel factor and self-organizing maps analyses
[J]. Bioresource Technology, 2019, 287: 121471. DOI: 10.1016/j.biortech.2019.121471.Characterization of biochar-derived dissolved organic matter using UV-visible absorption and excitation-emission fluorescence spectroscopies
[J]. Chemosphere, 2014, 103: 197-204. DOI: 10.1016/j.chemosphere.2013.11.066.Influence of leaching solution and catchment location on the fluorescence of water-soluble organic matter
[J]. Environmental Science and Technology, 2015, 49(7): 4425-4432. DOI: 10.1021/es504881t.Dissolved organic matter extracted with water and a saline solution from different soil profiles
[J]. Soil Science, 2010, 175(6): 255-262. DOI: 10.1097/ss.0b013e3181e457a6.Evaluating fluorescent dissolved organic matter released from wetland-plant derived biochar: Effects of extracting solutions
[J]. Chemosphere, 2018, 212: 638-644. DOI: 10.1016/j.chemosphere.2018.08.110.Quantification of chemical states, dissociation constants and contents of oxygen-containing groups on the surface of biochars produced at different temperatures
[J]. Environmental Science and Technology, 2015, 49(1): 309-317. DOI: 10.1021/es5043468.Surface chemistry variations among a series of laboratory-produced biochars
[J]. Geoderma, 2011, 163(3, 4): 247-255. DOI: 10.1016/j.geoderma.2011.04.021.Molecular-scale characterization of hot-water-extractable organic matter in organic horizons of a forest soil
[J]. Soil Science Society of America Journal, 2009, 73(3): 812-821. DOI: 10.2136/sssaj2008.0075.Field-scale fluorescence fingerprinting of biochar-borne dissolved organic carbon
[J]. Journal of Environmental Management, 2016, 169: 184-190. DOI: 10.1016/j.jenvman.2015.12.009.Evaluating soil dissolved organic matter extraction using three-dimensional excitation-emission matrix fluorescence spectroscopy
[J]. Pedosphere, 2017, 27(5): 968-973. DOI: 10.1016/S1002-0160(17)60466-1.The mechanism of p-nitrophenol degradation by dissolved organic matter derived from biochar
[J]. Science of the Total Environment, 2023, 868: 161693. DOI: 10.1016/j.scitotenv. 2023.161693.Study on the long-term effects of DOM on the adsorption of BPS by biochar
[J]. Chemosphere, 2020, 242: 125165. DOI: 10.1016/j.chemosphere.2019.125165.Interaction between biochar-dissolved organic matter and chlorophenols during biochar adsorption
[J]. Environmental Science and Pollution Research International, 2023, 30(14): 40375-40387. DOI: 10.1007/s11356-022-25083-1.Phototransformation of biochar-derived dissolved organic matter and the effects on photodegradation of imidacloprid in aqueous solution under ultraviolet light
[J]. Science of the Total Environment, 2020, 724: 137913. DOI: 10.1016/j.scitotenv.2020.137913.Application potential of biochar in environment: Insight from degradation of biochar-derived DOM and complexation of DOM with heavy metals
[J]. Science of the Total Environment, 2019, 646: 220-228. DOI: 10.1016/j.scitotenv. 2018.07.282.Adsorption of copper and zinc by biochars produced from pyrolysis of hardwood and corn straw in aqueous solution
[J]. Bioresource Technology, 2011, 102(19): 8877-8884. DOI: 10.1016/j.biortech.2011.06.078.Periodical changes of dissolved organic matter (DOM) properties induced by biochar application and its impact on downward migration of heavy metals under flood conditions
[J]. Journal of Cleaner Production, 2020, 275: 123787. DOI: 10.1016/j.jclepro.2020.123787.Elucidating the conformation effects within adsorption of natural organic matter on mesoporous graphitic carbon
[J]. Chemical Engineering Journal, 2024, 480: 148171. DOI: 10.1016/j.cej. 2023.148171.Utilization of UV-vis spectroscopy and related data analyses for dissolved organic matter (DOM) studies: A review
[J]. Critical Reviews in Environmental Science and Technology, 2017, 47(3): 131-154. DOI: 10. 1080/10643389.2017.1309186.Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon
[J]. Environmental Science and Technology, 2003, 37(20): 4702-4708. DOI: 10.1021/es030360x.Insight to the physiochemical properties and DOM of biochar under different pyrolysis temperature and modification conditions
[J]. Journal of Analytical and Applied Pyrolysis, 2022, 166: 105590. DOI: 10.1016/j.jaap.2022.105590.Estimation of the hydrophobic fraction of dissolved organic matter in water samples using UV photometry
[J]. Water Research, 2002, 36(20): 5037-5044. DOI: 10.1016/S0043-1354(02)00365-2.Molecular characterization of dissolved organic matter (DOM) along a river to ocean transect of the lower Chesapeake Bay by ultrahigh resolution electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry
[J]. Marine Chemistry, 2008, 110(3-4): 140-152. DOI: 10.1016/j.marchem.2008.04.008.Molecular insights into the transformation of dissolved organic matter in landfill leachate concentrate during biodegradation and coagulation processes using ESI FT-ICR MS
[J]. Environmental Science and Technology, 2017, 51(14): 8110-8118. DOI: 10.1021/acs.est.7b02194.Potential impact of biochar water-extractable substances on environmental sustainability
[J]. ACS Sustainable Chemistry and Engineering, 2013, 1(1): 118-126. DOI: 10.1021/sc300063f.Effects of biochar on microalgal growth: Difference between dissolved and undissolved fractions
[J]. ACS Sustainable Chemistry and Engineering, 2020, 8(24): 9156-9164. DOI: 10.1021/acssuschemeng.0c02768.Characterization of organic compounds in biochars derived from municipal solid waste
[J]. Waste Management, 2017, 67: 131-142. DOI: 10.1016/j.wasman.2017.05.052.Mercury complexation with dissolved organic matter released from thirty-six types of biochar
[J]. Bulletin of Environmental Contamination and Toxicology, 2019, 103(1): 175-180. DOI: 10.1007/s00128-018-2397-2.Molecular characterization of inhibiting biochar water-extractable substances using electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry
[J]. Environmental Science and Technology, 2013, 47(23): 13294-13302. DOI: 10.1021/es4034777.Characterization of mercury binding to different molecular weight fractions of dissolved organic matter
[J]. Journal of Hazardous Materials, 2022, 431: 128593. DOI: 10.1016/j.jhazmat.2022.128593.Dissolved organic matter in urban forestland soil and its interactions with typical heavy metals: A case of Daxing district, Beijing
[J]. Environmental Science and Pollution Research International, 2019, 26(3): 2960-2973. DOI: 10.1007/s113 56-018-3860-7.Effects of biochar on availability and plant uptake of heavy metals-A meta-analysis
[J]. Journal of Environmental Management, 2018, 222: 76-85. DOI: 10.1016/j.jenvman.2018.05.004.A review of biochar as a low-cost adsorbent for aqueous heavy metal removal
[J]. Critical Reviews in Environmental Science and Technology, 2016, 46(4): 406-433. DOI: 10.1080/106433 89.2015.1096880.A review on application of biochar in the removal of pharmaceutical pollutants through adsorption and persulfate-based AOPs
[J]. Sustainability, 2022, 14(16): 10128. DOI: 10.3390/su141610128.Biochar as a tool for effective management of drought and heavy metal toxicity
[J]. Chemosphere, 2021, 271: 129458. DOI: 10.1016/j.chemosphere.2020.129458.Characteristic of heavy metals in biochar derived from sewage sludge
[J]. Journal of Material Cycles and Waste Management, 2016, 18(4): 725-733. DOI: 10.1007/s10163-015-0366-y.Modified biochar: Synthesis and mechanism for removal of environmental heavy metals
[J]. Carbon Research, 2022, 1(1): 8. DOI: 10.1007/s44246-022-00007-3.Binding characteristics of copper onto biochar-derived DOM using general, heterospectral and moving-window two-dimensional correlation analyses
[J]. Journal of Hazardous Materials, 2022, 435: 129021. DOI: 10.1016/j.jhazmat.2022.129021.The effect of anthropogenic impoundment on dissolved organic matter characteristics and copper binding affinity: Insights from fluorescence spectroscopy
[J]. Chemosphere, 2017, 188: 424-433. DOI: 10.1016/j.chemosphere.2017.09.023.Effect of biochar-derived DOM on the interaction between Cu(II) and biochar prepared at different pyrolysis temperatures
[J]. Journal of Hazardous Materials, 2022, 421: 126739. DOI: 10.1016/j.jhazmat.2021.126739.Overlooked contributions of biochar-derived dissolved organic matter on the adsorption of Pb(Ⅱ): Impacts of fractionation and interfacial force
[J]. Journal of Hazardous Materials, 2021, 420: 126692. DOI: 10.1016/j.jhazmat.2021. 126692.Limited Cu(II) binding to biochar DOM: Evidence from C K-edge NEXAFS and EEM-PARAFAC combined with two-dimensional correlation analysis
[J]. Science of the Total Environment, 2020, 701: 134919. DOI: 10.1016/j.scitotenv.2019.134919.Effects of pyrolysis temperature on proton and cadmium binding properties onto biochar-derived dissolved organic matter: Roles of fluorophore and chromophore
[J]. Chemosphere, 2022, 299: 134313. DOI: 10.1016/j.chemosphere.2022. 134313.Characterization of copper binding to biochar-derived dissolved organic matter: Effects of pyrolysis temperature and natural wetland plants
[J]. Journal of Hazardous Materials, 2023, 442: 130076. DOI: 10.1016/j.jhazmat.2022. 130076.Analysis of the complexation behaviors of Cu(II) with DOM from sludge-based biochars and agricultural soil: Effect of pyrolysis temperature
[J]. Chemosphere, 2020, 250: 126184. DOI: 10.1016/j.chemosphere.2020.126184.Insight into metal binding properties of biochar-derived DOM using EEM-PARAFAC and differential absorption spectra combined with two-dimensional correlation spectroscopy
[J]. Environmental Science and Pollution Research International, 2021, 28(11): 13375-13393. DOI: 10.1007/s11356-020-11573-7.Biochars induced modification of dissolved organic matter (DOM) in soil and its impact on mobility and bioaccumulation of arsenic and cadmium
[J]. Journal of Hazardous Materials, 2018, 348: 100-108. DOI: 10.1016/j.jhazmat.2018.01.031.Combination of two-dimensional correlation spectroscopy and parallel factor analysis to characterize the binding of heavy metals with DOM in lake sediments
[J]. Journal of Hazardous Materials, 2013, 263: 412-421. DOI: 10.1016/j.jhazmat.2013.09.042.Insight into interactions of heavy metals with livestock manure compost-derived dissolved organic matter using EEM-PARAFAC and 2D-FTIR-COS analyses
[J]. Journal of Hazardous Materials, 2021, 420: 126532. DOI: 10.1016/j.jhazmat.2021.126532.Experimental and modeling study of proton and copper binding properties onto fulvic acid fractions using spectroscopic techniques combined with two-dimensional correlation analysis
[J]. Environmental Pollution, 2020, 256: 113465. DOI: 10.1016/j.envpol.2019.113465.Dynamic study of the lead (Pb) tolerance and accumulation characteristics of new dwarf bamboo in Pb-contaminated soil
[J]. Chemosphere, 2021, 282: 131089. DOI: 10.1016/j.chemosphere.2021.131089.Review of organic and inorganic pollutants removal by biochar and biochar-based composites
[J]. Biochar, 2021, 3(3): 255-281. DOI: 10.1007/s42773-021-00101-6.The binding properties of copper and lead onto compost-derived DOM using Fourier-transform infrared, UV–vis and fluorescence spectra combined with two-dimensional correlation analysis
[J]. Journal of Hazardous Materials, 2019, 365: 457-466. DOI: 10.1016/j.jhazmat.2018.11.035.Potential of dissolved organic matter (DOM) to extract As, Cd, Co, Cr, Cu, Ni, Pb and Zn from polluted soils: A review
[J]. Geoderma, 2019, 343: 235-246. DOI: 10.1016/j.geoderma. 2019.02.041.Relationship between heavy metals and dissolved organic matter released from sediment by bioturbation/bioirrigation
[J]. Journal of Environmental Sciences, 2019, 75: 216-223. DOI: 10.1016/j.jes.2018.03.031.Effects of pH and low molecular weight organic acids on competitive adsorption and desorption of cadmium and lead in paddy soils
[J]. Environmental Monitoring and Assessment, 2012, 184(10): 6325-6335. DOI: 10.1007/s10661-011-2422-y.Contribution of different mechanisms to Pb2+ and Cd2+ sorption on magnetic wheat straw biochars: Impact of pyrolysis temperature and DOM in biochar
[J]. Journal of Environmental Chemical Engineering, 2022, 10(3): 107851. DOI: 10.1016/j.jece.2022.107851.A review of arsenic reaction behavior in copper smelting process and its disposal techniques
[J]. Journal of Central South University, 2023, 30(8): 2510-2541. DOI: 10.1007/s11771-023-5392-7.Preparation of arsenic-antimony from arsenic alkali residue by calcification transformation-carbonthermal reduction
[J]. Journal of Central South University, 2023, 30(7): 2193-2204. DOI: 10.1007/s11771-023-5379-4.Soil contamination in China: Current status and mitigation strategies
[J]. Environmental Science and Technology, 2015, 49(2): 750-759. DOI: 10.1021/es5047099.Effect of dissolved organic carbon from sludge, rice straw and spent coffee ground biochar on the mobility of arsenic in soil
[J]. Science of the Total Environment, 2018, 636: 1241-1248. DOI: 10.1016/j.scitotenv.2018.04.406.Effects of biochar amendment on the availability of trace elements and the properties of dissolved organic matter in contaminated soils
[J]. Environmental Technology and Innovation, 2019, 16: 100492. DOI: 10.1016/j.eti.2019.100492.Degradation of tetracycline by nitrogen-doped biochar as a peroxydisulfate activator: Nitrogen doping pattern and non-radical mechanism
[J]. Sustainable Horizons, 2024, 10: 100091. DOI: 10.1016/j.horiz.2024.100091.Bisphenol A adsorption behavior on soil and biochar: Impact of dissolved organic matter
[J]. Environmental Science and Pollution Research International, 2021, 28(25): 32434-32445. DOI: 10.1007/s11356-021-12723-1.The role of dissolved organic matter during per- and polyfluorinated substance (PFAS) adsorption, degradation, and plant uptake: A review
[J]. Journal of Hazardous Materials, 2022, 436: 129139. DOI: 10.1016/j.jhazmat.2022.129139.Perturbation and strengthening effects of DOM on the biochar adsorption pathway
[J]. Ecotoxicology and Environmental Safety, 2022, 245: 114113. DOI: 10.1016/j.ecoenv.2022.114113.Effect of biochar-derived dissolved organic matter on adsorption of sulfamethoxazole and chloramphenicol
[J]. Journal of Hazardous Materials, 2020, 396: 122598. DOI: 10.1016/j.jhazmat.2020.122598.Sorption of sulfamethazine to biochars as affected by dissolved organic matters of different origin
[J]. Bioresource Technology, 2018, 248: 36-43. DOI: 10.1016/j.biortech. 2017.08.082.An innovative approach for landfill leachate treatment based on selective adsorption of humic acids with carbon nitride
[J]. Chemical Engineering Journal, 2023, 461: 142090. DOI: 10.1016/j.cej.2023.142090.Impacts of redox conditions on dissolved organic matter (DOM) quality in marine sediments off the River Rhône, Western Mediterranean Sea
[J]. Geochimica et Cosmochimica Acta, 2020, 276: 151-169. DOI: 10.1016/j.gca.2020.02.001.Application of carbon nitride nanosheets for adsorption of various humic substances from aqueous solutions
[J]. Chemical Engineering Journal, 2023, 454: 140296. DOI: 10.1016/j.cej.2022.140296.Interaction of bisphenol A with dissolved organic matter in extractive and adsorptive removal processes
[J]. Chemosphere, 2012, 87(8): 857-864. DOI: 10.1016/j.chemosphere.2012.01.026.Photochemical activity and characterization of the complex of humic acids with iron(III)
[J]. Journal of Geochemical Exploration, 2009, 102(2): 49-55. DOI: 10.1016/j.gexplo. 2009.02.003.Photochemistry of dissolved black carbon released from biochar: Reactive oxygen species generation and phototransformation
[J]. Environmental Science and Technology, 2016, 50(3): 1218-1226. DOI: 10.1021/acs.est. 5b04314.Controlled burning of forest detritus altering spectroscopic characteristics and chlorine reactivity of dissolved organic matter: Effects of temperature and oxygen availability
[J]. Environmental Science and Technology, 2015, 49(24): 14019-14027. DOI: 10.1021/acs.est.5b03961.Microbial community biomass and structure in saline and non-saline soils associated with salt- and boron-tolerant poplar clones grown for the phytoremediation of selenium
[J]. International Journal of Phytoremediation, 2018, 20(2): 129-137. DOI: 10.1080/15226514.2017.1337073.Soil microbial community structure affected by biochar and fertilizer sources
[J]. Applied Soil Ecology, 2020, 150: 103452. DOI: 10.1016/j.apsoil.2019.103452.Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: A review
[J]. Environmental Pollution, 2017, 227: 98-115. DOI: 10.1016/j.envpol.2017.04.032.Enhanced bioreduction of iron and arsenic in sediment by biochar amendment influencing microbial community composition and dissolved organic matter content and composition
[J]. Journal of Hazardous Materials, 2016, 311: 20-29. DOI: 10.1016/j.jhazmat.2016.02.069.Biochar effects on soil biota–A review
[J]. Soil Biology and Biochemistry, 2011, 43(9): 1812-1836. DOI: 10.1016/j.soilbio.2011.04.022.Taxa-specific changes in soil microbial community composition induced by pyrogenic carbon amendments
[J]. Soil Biology and Biochemistry, 2011, 43(2): 385-392. DOI: 10.1016/j.soilbio.2010.11.005.How biochar works, and when it doesn’t: A review of mechanisms controlling soil and plant responses to biochar
[J]. GCB Bioenergy, 2021, 13(11): 1731-1764. DOI: 10.1111/gcbb. 12885.Abundant and stable char residues in soils: Implications for soil fertility and carbon sequestration
[J]. Environmental Science and Technology, 2012, 46(17): 9571-9576. DOI: 10.1021/es30 1107c.Quantification and characterization of dissolved organic carbon from biochars
[J]. Geoderma, 2019, 335: 161-169. DOI: 10.1016/j.geoderma.2018.08.019.Chemical composition and potential bioactivity of volatile from fast pyrolysis of rice husk
[J]. Journal of Analytical and Applied Pyrolysis, 2015, 112: 394-400. DOI: 10.1016/j.jaap.2015. 02.021.Water extract from straw biochar used for plant growth promotion: An initial test
[J]. BioResources, 2015, 11(1): 249-266. DOI: 10.15376/biores.11.1.249-266.Induction of systemic resistance in plants by biochar, a soil-applied carbon sequestering agent
[J]. Phytopathology, 2010, 100(9): 913-921. DOI: 10.1094/PHYTO-100-9-0913.Determinants of Pseudomonas putida WCS358 involved in inducing systemic resistance in plants
[J]. Molecular Plant Pathology, 2005, 6(2): 177-185. DOI: 10.1111/j.1364-3703. 2005.00276.x.Carbon controls on spodosol nitrogen, sulfur, and phosphorus cycling
[M]//Carbon Forms and Functions in Forest Soils.Transport and fractionation of dissolved organic matter in soil columns
[J]. Soil Science, 2003, 168(2): 108-118. DOI: 10.1097/00010694-200302000-00005.Transport of dissolved organic matter through a sandy forest soil
[J]. Soil Science Society of America Journal, 1997, 61(3): 920-927. DOI: 10.2136/sssaj1997.03615995006100030030x.Multiple exchange processes on mineral surfaces control the transport of dissolved organic matter through soil profiles
[J]. Soil Biology and Biochemistry, 2018, 118: 79-90. DOI: 10.1016/j.soilbio.2017.12.006.Estimates of annual leaching losses of dissolved organic carbon from pastures on Allophanic Soils grazed by dairy cattle, Waikato, New Zealand
[J]. New Zealand Journal of Agricultural Research, 2016, 59(1): 32-49. DOI: 10.1080/00288233.2015.1120222.Amending greenroof soil with biochar to affect runoff water quantity and quality
[J]. Environmental Pollution, 2011, 159(8, 9): 2111-2118. DOI: 10.1016/j.envpol.2011.01.022.Microbial degradation of dissolved organic matter (DOM) and its influence on phenanthrene–DOM interactions
[J]. Chemosphere, 2011, 85(8): 1360-1367. DOI: 10.1016/j.chemosphere.2011.08.001.Changes in the molecular composition of organic matter leached from an agricultural topsoil following addition of biomass-derived black carbon (biochar)
[J]. Organic Geochemistry, 2014, 69: 52-60. DOI: 10.1016/j.orggeochem.2014.02.003.Effects of laboratory biotic aging on the characteristics of biochar and its water-soluble organic products
[J]. Journal of Hazardous Materials, 2020, 382: 121071. DOI: 10.1016/j.jhazmat.2019.121071.A comprehensive assessment of potential hazard caused by organic compounds in biochar for agricultural use
[J]. Journal of Hazardous Materials, 2021, 403: 123644. DOI: 10.1016/j.jhazmat.2020.123644.A comprehensive review of biochar-derived dissolved matters in biochar application: Production, characteristics, and potential environmental effects and mechanisms
[J]. Journal of Environmental Chemical Engineering, 2021, 9(3): 105258. DOI: 10.1016/j.jece.2021.105258.Production temperature effects on the structure of hydrochar-derived dissolved organic matter and associated toxicity
[J]. Environmental Science and Technology, 2018, 52(13): 7486-7495. DOI: 10.1021/acs.est.7b04983.Potential effects of biochar on the availability of phosphorus—Mechanistic insights
[J]. Geoderma, 2016, 277: 83-90. DOI: 10.1016/j.geoderma.2016.05.007.ZHANG Xiu-xiu, NAN Hong-yan, LIU Gong-gang, SHOW Pau-Loke, and WANG Chong-qing declare that they have no conflict of interest.
ZHANG Xiu-xiu, NAN Hong-yan, LIU Gong-gang, SHOW Pau-Loke, WANG Chong-qing. A review on dissolved organic matter derived from biochar in soil: Characterizations, interaction with pollutants, and transformation [J]. Journal of Central South University, 2025, 32(1): 122-148. DOI: https://doi.org/10.1007/s11771-025-5861-2.
张秀秀,南红岩,刘贡钢等.土壤中生物炭溶解有机质的研究进展:表征、污染物相互作用及转化[J].中南大学学报(英文版),2025,32(1):122-148.