1 Introduction
As is well known, the steel industry is an energy consumption sector and a huge emission source of greenhouse gas and various toxic pollutants, including SO2, NOx, etc [1-3]. Statistical data showed that the CO2 emitted from steelworks exceeded 15% of total CO2 emission from Chinese industry and even accounts for about 30% of that from major cities [1, 4]. Consequently, it is of great importance to abate the CO2 emissions in the steelmaking sector to meet the domestic low carbon development policy in the coming decades.
Iron ore sintering is an essential process with its purpose to prepare the lumpy feeding materials for ironmaking in the blast furnace. The CO2 emitted in sintering and blast furnace contributed about 15% and 70% of the total CO2 emission from the whole steelmaking flow, respectively [5]. To reduce the CO2 emitted by the entire iron and steelmaking, a new process, i.e. using pre-reduced iron ore as raw materials in ironmaking was proposed in Japan [6]. JFE Iron and Steel Corporation of Japan has carried out some research on the use of pre-reduced iron ore which was generated by a fluidized bed reduction as raw materials for ironmaking [6]. The result showed that the energy consumption of blast furnace was significantly decreased through the use of pre-reduced iron ores as burden materials. YABE et al [7] pre-reduced two brands of iron ores by fluidized bed reduction with blast furnace gas and then used them in a sinter pot test. Their results confirmed that using pre-reduced iron ore as a sinter raw material enabled CO2 abatement not only in the ironmaking process but also in the whole steelworks. SATOSHI [8] developed a pre-reduction sintering process (PSP), which achieved the agglomeration and partial reduction of fine iron ores simultaneously in the sintering process. They used the pre-reduced agglomerates in blast furnace and found that the total CO2 emission from the entire iron-making process also showed a downward trend, and the utilization efficiency of coke breeze was significantly improved [8]. ZHOU et al [9] indicated that raw materials of pre-reduction sintering with higher iron grades might achieve a better metallization ratio of pre-reduced sinter. Simultaneously, with the increase of the content of solid carbon added in PSP, the reducibility of pre-reduced sinter was improved.
With the development of steel production in China, apart from the serious environmental issue caused by CO2 emission, another outstanding question is the increasingly aggravated exhaustion of high-grade iron ore resources. In order to meet the sustainable development of iron and steel industry, abundant iron ores were imported. However, the gradual decrease of iron grade and increase of harmful elements, such as alkali metal elements, in imported iron ore have been observed. The increasing contents of alkali metals will aggravate the pulverization of sinter products and cause the abnormal expansion of pellet ore, adversely affecting the blast furnace smelting [10-13]. Moreover, alkali metals greatly reduced the thermal strength of coke and exacerbated coke pulverization, resulting in worse material layer permeability of blast furnace. Furthermore, the cyclic enrichment of alkali metals in blast furnace will induce the occurrence of blast furnace nodules and furnace lining erosion. All of these problems deriving from alkali metals will lead to a deterioration in the furnace condition, a decrease in its productivity, an increase in coke ratio, and even a damage in the smooth running of blast furnace [14-17].
For the steel industry in China, sintering products about 1 billion tons per year take up around 75% of the total iron-bearing burden of ironmaking [18]. Studies have reported that over 80% (mass fraction) of K and Na would enter the blast furnace with a sinter [19-21]. Hence, it is of the essence to reduce the contents of K and Na from the sinter. There have been currently two main processes for K and Na removal from blast furnace burden, i.e., iron concentrate flotation and chlorination sintering processes [22-25]. The former as the main measure of removing K and Na from crude iron ore has some effectiveness. However, it also possesses the disadvantages of high contents of residual alkali metal, poor adaptability to different iron ores, and complex technological process. The chlorination sintering was another effective technology for K and Na removal. Its principle is to use the difference in chemical stability between KCl, NaCl and metal chlorides such as CaCl2(MgCl2) [10]. In this way, Ca2+ replaced K+ and Na+ in sinter or pellet ore to generate KCl and NaCl with lower melting point, higher volatility and reducibility, which can be volatilized at high temperature and taken away by exhaust gas flow. Nevertheless, the cold strength of product sinter was reduced and the cost of CaCl2 was high [26]. Therefore, it is urgent to provide alternatives to alleviate or even completely solve the harm of alkali metal to blast furnace smelting.
In this paper, a pre-reduction sintering process was employed to achieve alkali metal element removal. Recirculating CO-containing flue gas into sintering bed was also performed, which not only improved the removal of K and Na but also contributed to realizing the value-added utilization of wasted flue gas. The results obtained from this study indicated that the pre-reduction sintering process with flue gas recirculation can simultaneously enable the efficient removal of K and Na, and the reduction of flue gas emission in whole steelworks.
2 Experimental
2.1 Materials
The raw materials for sintering pot tests including iron concentrate, fluxes (quicklime, limestone and dolomite) and coke breeze, were taken from Bayan Obo, China. Table 1 gives the detailed chemical compositions of raw materials. The iron concentrate had an iron content of 64.18%. The contents of K and Na in iron concentrate were high, reaching 0.10% and 0.21%, respectively. The contents of SiO2, CaO, Al2O3, MgO and LOI were separately 2.79%, 0.72%, 0.43%, 0.63% and 1.95%. The basicity (CaO/SiO2, mass%) of the raw material mixture was controlled at 1.80 by adjusting the addition of fluxes. The experiments in which the coke breeze added ranged from 5% to 13% were researched to elucidate the effect of coke breeze dosage on traditional sintering process (TSP) and PSP. The proportion of return fines was 30.00%.
| Material | TFe | SiO2 | CaO | MgO | Al2O3 | K2O | Na2O | LOI |
|---|---|---|---|---|---|---|---|---|
| Concentrate | 64.18 | 2.79 | 0.72 | 0.63 | 0.43 | 0.10 | 0.21 | 1.95 |
| Return fine | 58.56 | 4.02 | 9.83 | 2.07 | 1.88 | 0.06 | 0.08 | 0.11 |
| Quicklime | 0.64 | 2.69 | 63.11 | 3.11 | 0.11 | 0.08 | 0.03 | 20.76 |
| Limestone | 0.35 | 2.63 | 51.27 | 2.85 | 0.11 | 0.13 | 0.03 | 42.76 |
| Dolomite | 1.51 | 3.77 | 34.16 | 21.33 | 0.03 | 0.12 | 0.04 | 5.68 |
| Coke breeze | 1.74 | 7.11 | 0.35 | 0.08 | 1.82 | 0.12 | 0.09 | 85.87 |
Table 2 documents the size distribution of iron concentrate. It can be found that iron concentrate with particle size greater than 0.074 mm accounted for 5.5%, and smaller than 0.045 mm accounted for 80.95%. More than 70% of fluxes and fuel were less than 3 mm satisfying the requirement of sinter production [27].
| Ore type | Size distribution | ||
|---|---|---|---|
| >0.074 mm | 0.045-0.074 mm | <0.045 mm | |
| Concentrate | 5.50 | 13.55 | 80.95 |
2.2 Methods
2.2.1 Granulation test
According to the test project, iron concentrates, return fines, fuel and fluxes were proportioned and mixed uniformly with prescribed moisture. Then, the mixture was charged into a granulation drum (600 mm in diameter and 1400 mm in length) at 15 r/min to granulate the fine particles into moist granules. There were two granulating methods in this study: conventional granulation and fuel segregated granulation. The difference between them was the order of the addition of fuel during granulation. Conventional granulation was to mix iron concentrates, return fines, fuel and fluxes together with water and then fed the mixture into a drum mixer for 5 min. Fuel segregated granulation involved granulating all the iron ores and fluxes as well as part of the coke breeze (0%, 20%, 40% and 60% of the total coke breeze added) for 2 min, and then the rest of coke breeze was added for the secondary granulation for 4 min.
2.2.2 Sintering trials
As shown in Figure 1, sintering tests were conducted in a laboratory-scale sintering pot with dimensions of 200 mm in diameter and 500 mm in height. 1 kg sinter with a diameter of 10-16 mm was placed on the grate at the bottom of the sinter pot as a hearth layer of 20-30 mm in height, and the granulated mixture was then fed in. After feeding, the granules in the surface layer were ignited by an ignition hood for 1.5 min at (1100±50) ℃. The suction pressure of ignition and sintering were respectively controlled at 5 and 10 kPa. The maximum temperature of flue gas measured by a B-type thermocouple suggested the endpoint of sintering process. The compositions of flue gas during sintering process were tested by an MGA-5 flue gas analyzer produced by MRU Corporation of Germany. Sintering pot tests were repeated 3 times for the experimental reproducibility and reliability.
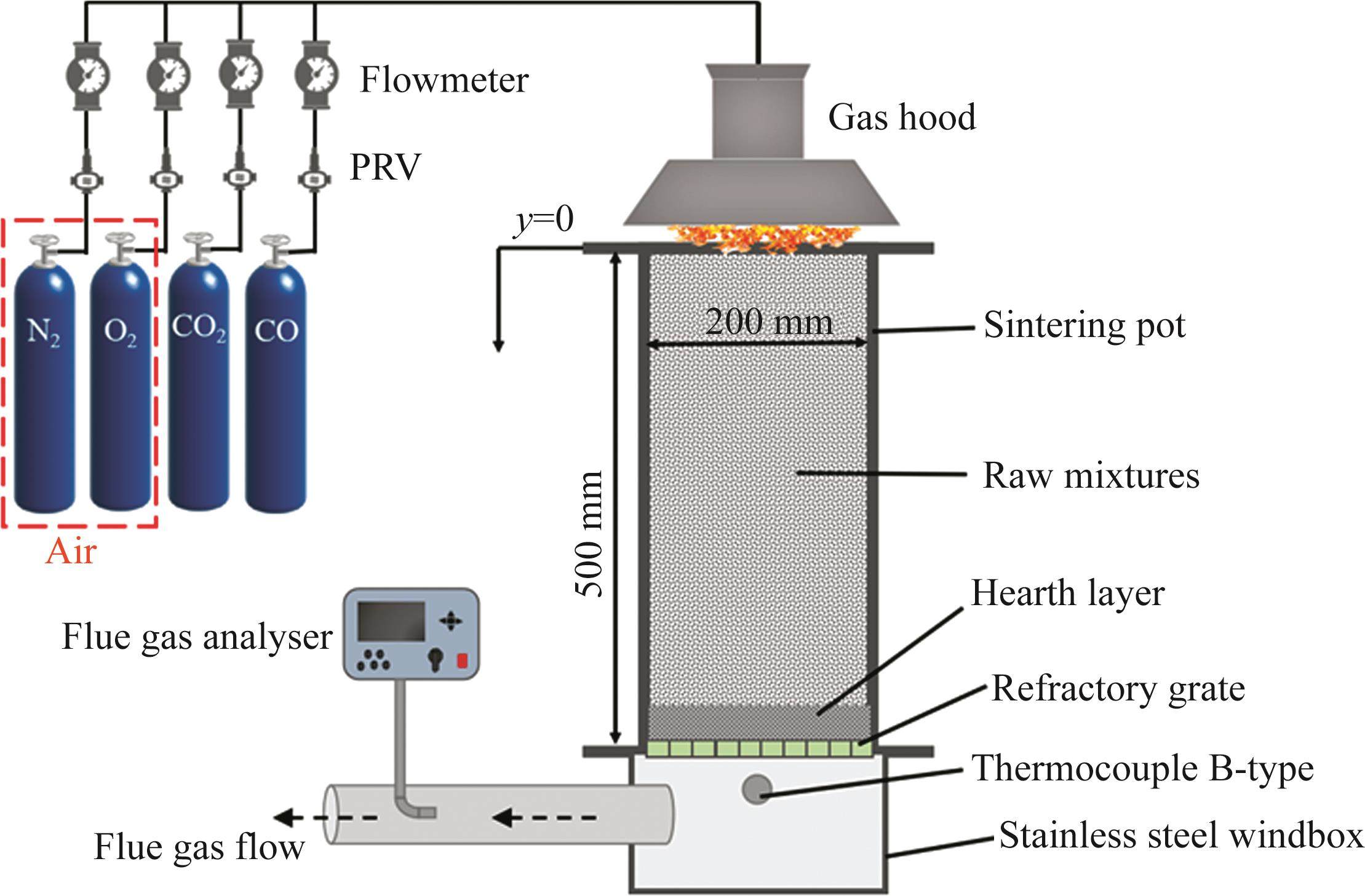
The gaseous medium for sintering process without flue gas recirculation (FGR) was air which mainly consisted of 21% O2 and 79% N2. For simulated FGR sintering process, the composition of gaseous medium was changed. As illustrated in Figure 1, the mixed gas of purified gases (O2, N2, CO2 and CO) was introduced directly to the surface of sintering bed through a gas hood added to the top of sinter pot after igniting. During the whole sintering process, the components of flue gas was detected by a flue gas analyzer, which can examine the real time concentration of O2, CO2, CO, NOx, SO2, etc.
2.2.3 Evaluation indexes
The evaluation indexes of sintering quality included sintering speed, sinter yield, tumbler index and productivity were calculated as follows in Table 3. The removal ratios of K and Na were calculated via Eq. (1).

| Evaluation index | Formula | Note |
|---|---|---|
Sintering speed, V⊥/(mm·min-1) | 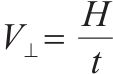 | H—initial height of sintering bed, mm; t—sintering time, min; sintering time—period of flue gas temperature reaching the highest point after ignition. |
| Yield, Y/% | 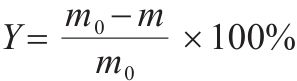 | m0—mass of sample with hearth layer deducted, kg; m—mass of >5 mm sample, kg. |
Productivity, P/(t·m-2·h-1) | 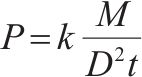 | M—mass of finished sinter, kg; D—diameter of pot, mm; k—conversion factor, 7.65×104; t—sintering time, min. |
| Tumbler index, TI/% | 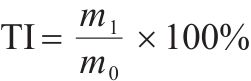 | m0—mass of sample with hearth layer deducted, kg; m1—mass of >6.3 mm sample after tumble, kg. |
where R is the removal ratio of K and Na, wt%; cs and cr are the contents of K and Na in sinter and raw mixtures respectively, mg/kg; ms and mr are the total mass of sinter and raw mixture, kg.
The metallization ratio was calculated by using the following formula.
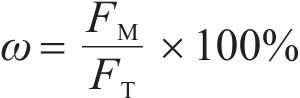
where ω is the metallization ratio; FM is the metallic iron content (wt%) in the sinter; FT is the total iron content (wt%) in the raw mixtures.
2.3 Analysis and characterization
The contents of hazardous elements K and Na in raw mixtures and finished sinter were examined by Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-AES Optima 30, JobinYvon, France) after acid digestion. Optical microstructure images of sinters were taken by an electric focusing front polarizing microscope (OM, Leica DMREX, DEU). The images at different magnifications were captured and analyzed by Leica image analysis software (Qwin) according to different gray value settings. Mineral phases showed different colors and shapes, which can be used to distinguish them from each other. The thermodynamic analysis was calculated by Factsage 7.0.
3 Results and discussion
3.1 Theoretical analysis
3.1.1 Reduction thermodynamics of K and Na
Alkalies are generally introduced into the furnace through the raw materials, in the form of silicates, oxides, carbonates and halides [28]. The relationships between the standard free energy of formation of potassium and sodium compounds that may occur in the sintering process and temperature were shown in Figure 2. It can be seen from this figure that, at standard atmospheric pressure, K2O and Na2O can be reduced by solid carbon at temperature above 815 and 1000 ℃, respectively, according to Eqs. (3) and (4). Alkali carbonates are more stable than alkali oxides, and at temperature higher than 1200 ℃, alkali carbonates can be reduced by solid carbon as shown in Eqs. (5) and (6). As also can be seen from Figure 2, at standard atmospheric pressure, the reduction of K2SiO3 by solid carbon can take place when the temperature is above 1500 ℃ (Eq. (7) or (8)), and the corresponding temperature for the reduction of Na2SiO3 is about 1700 ℃ (Eq. (9) or (10)). The temperature in TSP is 1000-1300 ℃, while the temperature in PSP is higher than that in TSP and the solid carbon in PSP is sufficient due to the excessive addition of coke breeze. Therefore, from the view of thermodynamics, the removal of alkali metals (K and Na) from the sinter through PSP is feasible.

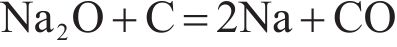

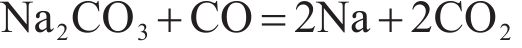



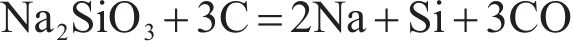

3.1.2 Reduction thermodynamics of iron oxides
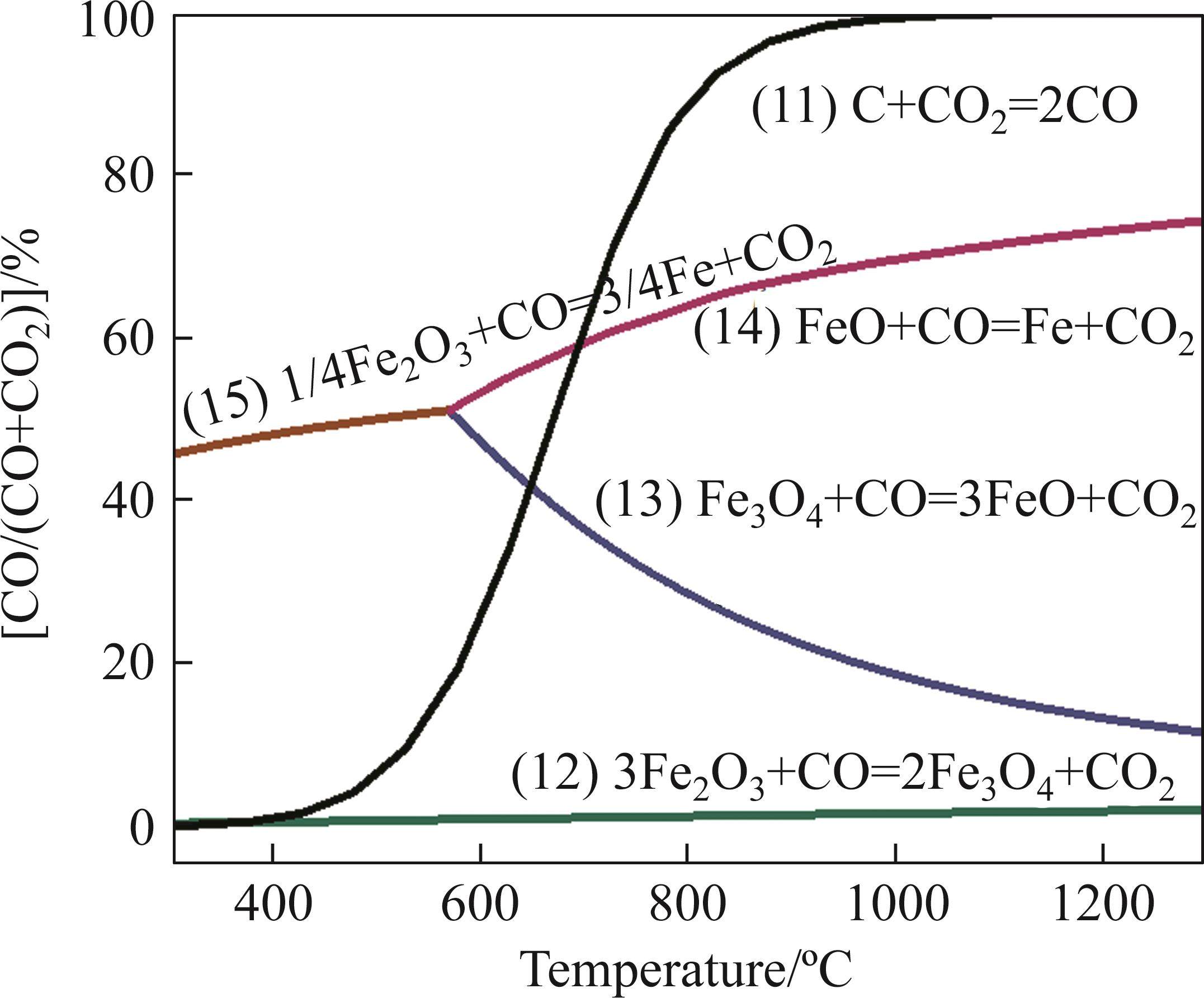
The main reactions during reduction process of iron oxides and corresponding phase equilibrium diagram are presented in Table 4 and Figure 3, respectively. Figure 3 shows that the reduction of iron oxides in sintering is closely related to the temperature and CO concentration. According to previous research reported [29], the increase of solid fuel addition promoted the CO/(CO+CO2) value and enhanced the local reduction atmosphere of sintering bed. The increase of CO/(CO+CO2) value facilitated the reactions of Eqs. (12) and (14). Moreover, the sintering temperature rose with the increase of fuel consumption, which further improved the development of Eqs. (12) and (14). Therefore, PSP has better conditions for the reduction of Fe3O4 to FeO and even can obtain the direct reduction iron.
| Equation | Reaction | ΔGΘT/(J·mol-1) |
|---|---|---|
| (11) |  |  |
| (12) |
|  |
| (13) |
|  |
| (14) |
| 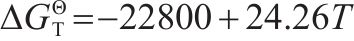 |
| (15) |
| 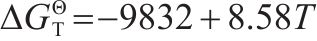 |
3.2 Traditional sintering process
Figures 4(a) and (b) give the effects of moisture content of mixture (constant 5.5% coke dosage) and coke dosage (constant 8% moisture) on sinter indexes of TSP. From Figure 4(a), it can be observed that with moisture content rising, sintering speed and productivity were both improved, especially the moisture content risen from 7% to 8%. However, Tumbler index was reduced with the moisture content rising. Increasing coke dosage led to the decrease of sintering speed (Figure 4(b)). This was because higher coke dosage made the burning layer thicker which gives rise to the poor bed permeability, and thus the sintering speed decreased [30]. Moreover, it also can be seen from Figure 4(b) that tumbler index reached the maximum at the moisture content of 8% and coke dosage of 5.5%, respectively. As a consequence, the appropriate conditions for TSP were established as follows: moisture content 8% and coke dosage 5.5%. The sinter indexes of yield, sintering speed, productivity and tumbler index under the conditions were separately 68.68%, 21.61 mm/min, 1.53 t/(m2·h) and 65.70%. As displayed in Figure 4(c), under the optimal conditions, the K and Na removal ratios were respectively 15.63% and 21.36%, and the contents of K and Na in sinter were separately 0.084% and 0.14%. The reason for the partial removal of K and Na by TSP can be described as the temperature of the lower sinter layer increased due to the self-accumulating effect, thereby achieving the removal of K and Na, or the decomposition and removal of low melting point potassium and sodium compounds. The K2O and Na2O can be reduced by solid carbon, and the alkali carbonates can be partially reduced by solid carbon at the TSP sintering temperature. The reaction products of K and Na were insufflated in the dust removal system by airflow.

However, the alkali contents in sinter product were still high. In this study, the alkali content (K2O+Na2O) in traditional sinter reached 0.29%, which was higher than its threshold of 0.2% in Chinese industry standard (YB/T 4803—2020). Therefore, to increase the removal ratio of K and Na for alleviating their harmful effect on blast-furnace ironmaking production, the pre-reduction sintering process (PSP) was proposed.
3.3 Pre-reduction sintering process
3.3.1 Effect of coke dosage on PSP
Figure 5(a) displays the effect of coke dosage on removal ratios of K and Na and metallization ratio during PSP. It can be seen from the figure that the removal ratios of K and Na and metallization ratio all increased with the increase of coke dosage at first, and then decreased when coke dosage was higher than 12%. The effect of coke dosage on sintering indexes is indicated in Figure 5(b). As can be seen from Figure 5(b), with the increase of coke breeze dosage from 10% to 13%, the sintering speed and productivity showed a downward trend, which decreased from 18.72 mm/min and 1.36 t/(m2·h) to 16.12 mm/min and 1.16 t/(m2·h), respectively. By contrast, the yield and tumbler index were first increased and then slightly decreased as coke dosage increased. The yield and tumbler index reached their maximum value at coke dosage of 11.0% and 12.0%, respectively. From the above, the appropriate coke dosage was chosen as 12.0% for PSP.

The removal ratios of K and Na in PSP at coke dosage 12% were 54.03% and 30.28%, respectively, which were much higher than those in TSP. It was because the temperature in PSP is higher than that in TSP and the solid carbon in PSP is sufficient. The residual K and Na contents in pre-sinter were separately 0.045% and 0.12%. The alkali content (K2O+Na2O) in pre-reduced sinter was 0.22%. Further research on enhanced pre-reduction sintering technology for K and Na removal is needed. It can be known from thermodynamics in Figure 2 that the reduction of alkali metal silicates required a higher temperature. Hence, raising sintering temperature may be a possible method.
3.3.2 Effect of fuel segregated granulation
According to the work of OGI et al [31], coke coating granulation can improve the coke combustibility. Actually, the optimization of solid fuel distribution in granules is an effective measure to enhance the fuel combustion efficiency. Figure 6 shows the schematic illustration of different fuel distribution states in granules. Conventionally, the iron ores, fluxes and fuels are granulated together with water added. Hence, as illustrated in Figure 6, fuels were distributed equably in the granules. With fuel segregated granulation, the structure of quasi-particle was divided into interior layer and exterior layer. As displayed in Figure 6, part of coke breeze was wrapped inside and uniformly distributed in interior layer, and the rest was coated to the exterior layer. The proportion of coke breeze in the two parts directly decided the degree to which the fuel was wrapped. Accordingly, the out-proportioning ratio of coke breeze (defined as the proportion in the exterior layer of the granules) during fuel segregated granulation is a significant parameter. As the out-proportioning ratio increased, the degree to which the fuel was encapsulated decreased.

Figure 7(a) shows the removal ratios of K and Na as well as the metallization ratio during PSP (coke breeze dosage 12.0%) when the out-proportioning ratios of coke breeze were 0%, 20%, 40% and 60%. As can be seen from this figure, the fuel segregated granulation had a significant effect on the improvement of K and Na removal ratios and metallization ratio. With the increase of out-proportioning ratio of coke breeze from 0% to 60%, K removal efficiency increased from 54.00% to 58.00%, along with an increase in metallization ratio of sinter from 0.42% to 1.29%. The removal ratio of Na marginally increased with the rise of out-proportioning ratio of coke breeze. The influence of out-proportioning ratio of coke breeze on sinter indexes was indicated in Figure 7(b). Sintering speed and productivity were separately about 17.48 mm/min and 1.23 t/(m2·h) with conventional granulation (out-proportioning ratio of coke breeze 0%). When the out-proportioning ratio increased, sintering speed and productivity both dropped slightly. The yield of the sinter increased compared with that of conventional granulation, and it increased from 73.36% to 74.39% when the out-proportioning ratio of coke breeze increased from 0% to 60%. As for tumbler index, it was optimal when the out-proportioning ratio of coke breeze was 20%. So, from the comprehensive consideration of K and Na removal efficiencies, metallization ratio and sintering indexes, the optimal out-proportioning ratio of coke breeze was chosen to be 60%.
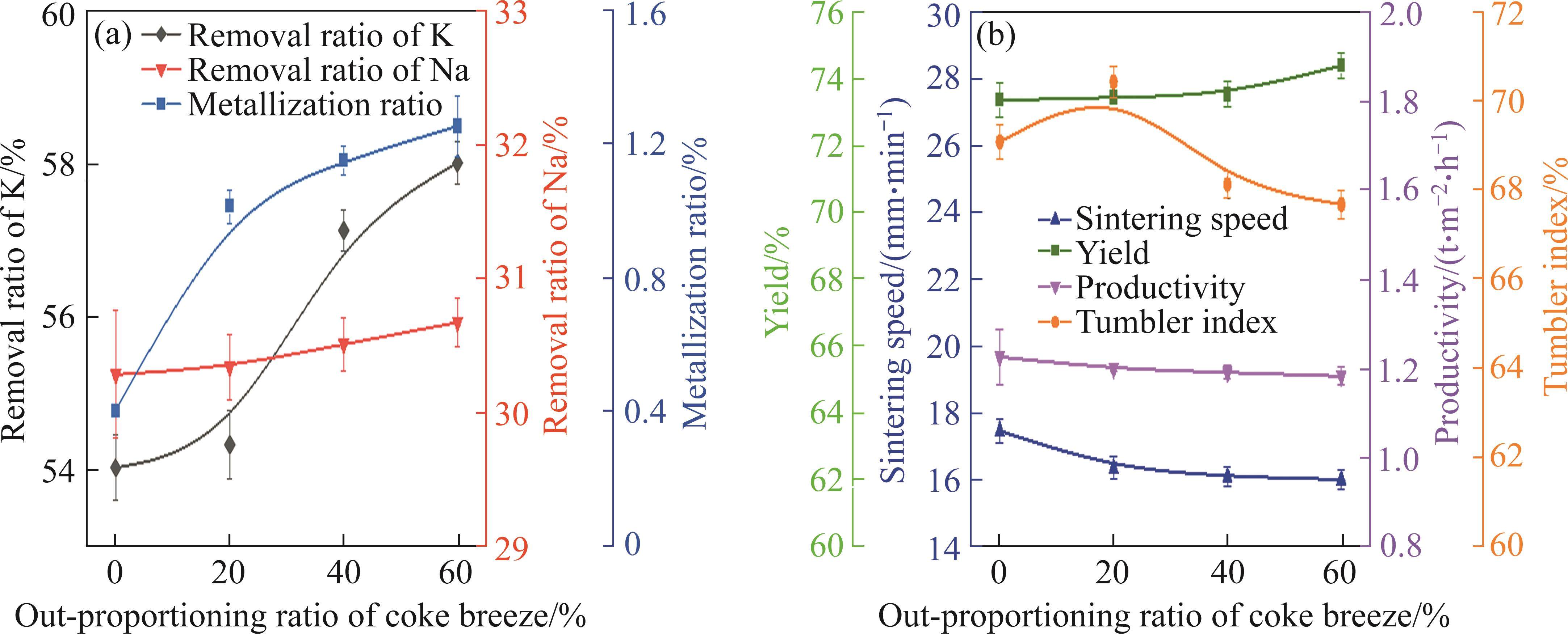
The conventional granulation will cause the fuel to be wrapped deeply, which hinders the passage of gas and affects the combustion and gasification reaction of fuel particles. Proportioning part of coke breeze in the exterior layer of granules helps the contact of fuels with oxygen and makes the combustion properly concentrated, which can increase the maximum temperature in the sintering process. The generated CO2 in the combustion process of coated coke breeze diffused inward and gasification reaction between CO2 and internal coke particles occurred, which produced more CO in the inner layer. Therefore, the inner part of the granules in fuel segregated granulation was characterized by a stronger reducing atmosphere than that in conventional granulation.
3.3.3 Comparison of TSP and PSP
3.3.3.1 Sintering indexes
Table 5 shows the comparison of K and Na removal ratios, metallization ratio and sinter indexes between TSP and PSP with fuel segregated granulation (PSPfsg) under the corresponding optimal conditions. Due to stronger reductive atmosphere and higher temperature in the sintering bed, K and Na removal ratios of PSPfsg were much higher than that of TSP, reaching 58.02% and 30.68%, respectively. Meanwhile, compared with that of TSP, the yield and tumbler index significantly improved to 74.40% and 68.69% respectively, while the productivity decreased to 1.18 t/(m2·h). The alkali content (K2O+Na2O) was 0.19%, which was lower than the threshold in Chinese industry standard (YB/T 4803—2020).
| Sintering process | K removal ratio/% | Na removal ratio/% | K content in sinter/% | Na content in sinter/% | Yield/% | Productivity/ (t·m-2·h-1) | Tumbler index/% |
|---|---|---|---|---|---|---|---|
| TSP | 15.63 | 21.36 | 0.084 | 0.14 | 67.68 | 1.53 | 65.70 |
| PSPfsg | 58.02 | 30.68 | 0.042 | 0.11 | 74.40 | 1.18 | 68.69 |
3.3.3.2 Emission characteristics of sintering flue gas
The comparison of concentration-time profiles of CO, CO2, O2, SO2 and NOx in exhaust gas during TSP and PSPfsg were displayed in Figure 8. As can be seen from Figure 8, the sintering time in PSPfsg was longer than that in TSP. Moreover, from
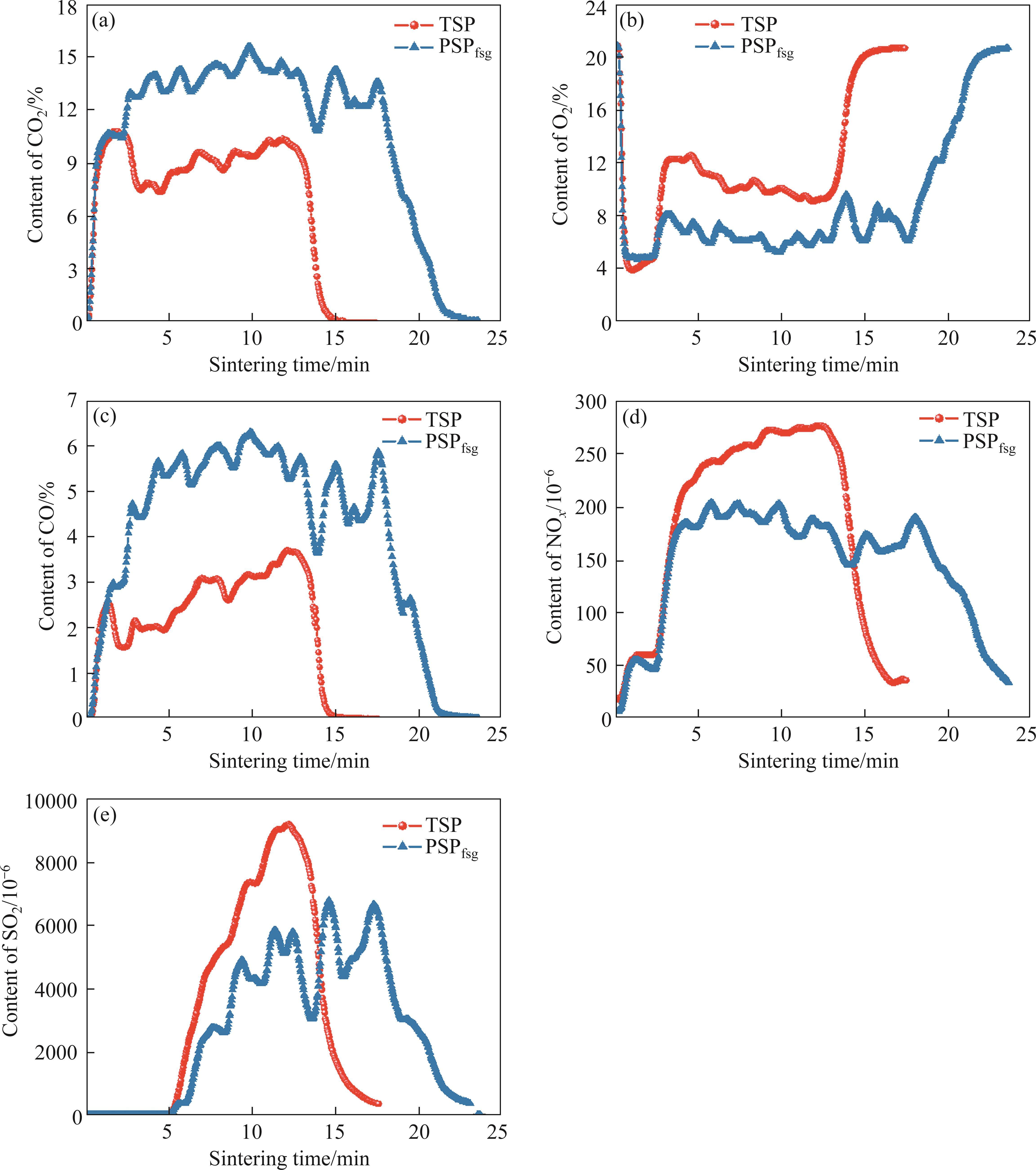
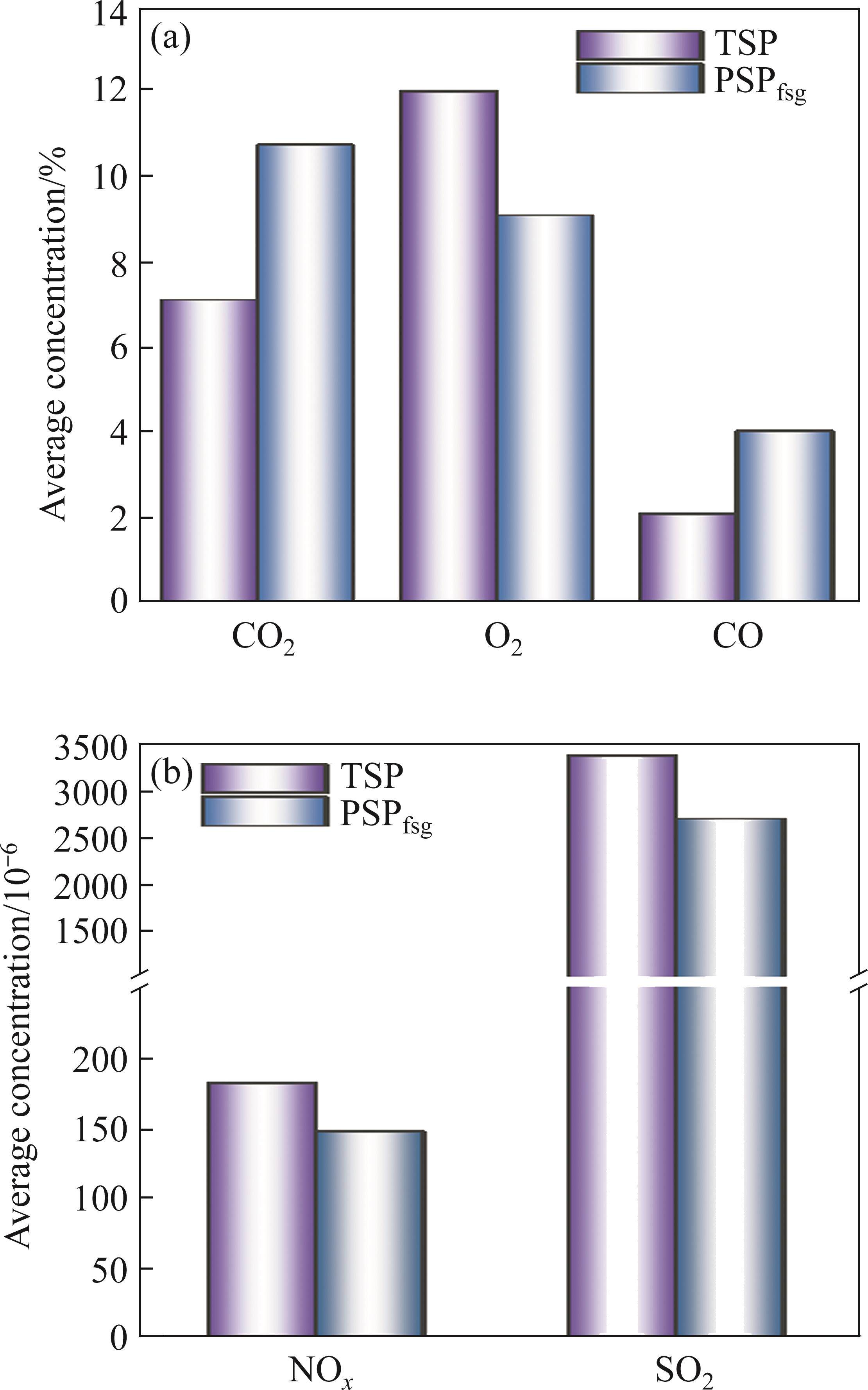
Figures 8(a)-(c), we can see that during PSPfsg, O2 content reduced in comparison with TSP due to the combustion of excess coke breeze while the contents of CO and CO2 had opposite rules, which contributed to better reducing atmosphere. Hence, the removal ratios of K and Na and metallization ratio improved in PSPfsg. It can be seen from Figures 8(d) and (e) that SO2 and NOx generated in PSPfsg decreased to a certain extent. Based on the data in Figure 8, the average concentration of these gas components was calculated, as shown in Figure 9. Compared with the TSP, the maximum growths of released CO2 and CO were approximately 4% and 2%, respectively. Nevertheless, the average concentration of NOx and SO2 both dropped to below 200×10-6 and 3000×10-6, respectively. These indicated that PSP was conducive to reducing pollutant gases including NOx and SO2 emissions and simultaneously increasing CO content. However, the CO2 emission in PSPfsg increased because of the excessive addition of coke breeze.
3.3.4 Optimization of PSPfsg by flue gas recirculation
According to the results in Section 3.3.3, the sintering quality in PSPfsg had already been higher than that in TSP. However, due to the higher coke breeze addition, PSPfsg would produce more CO and CO2 emission in flue gas compared with TSP. Direct emission of flue gas during PSPfsg not only polluted the environment, but also led to a waste of resources. In addition, compared with air, sintering flue gas was characterized by lower O2 content, higher CO content and higher temperature. Its recirculation could provide a better reducing atmosphere for PSPfsg and prevent the reoxidation of reduced iron in the sinter zone in the upper material layer.
Figure 10 demonstrated the effect of adding CO into air on K and Na removal ratios and sintering indexes with gas flow of 10 m3/min. It can be seen from Figure 10(a) that K and Na removal ratios and metallization ratio of sinter can be significantly improved by adding a certain amount of CO in the sintering process. When CO content increased to 5%, the removal ratios of K and Na reached the maximum of 74.11% and 32.92%, respectively, and the metallization ratio rose to 5.86%. Fortunately, literature studies have showed that for every 10% of increase in metallization ratio in sinter, there was a decrease of 4%-7% in coke ratio of blast furnace on average [6, 32]. In consequence, PSPfsg can effectively reduce the total CO2 emissions in the process of sintering-blast furnace ironmaking.

It can be found from Figure 10(b) that with the content of CO increasing, the productivity and sintering speed appeared a decrease, while the yield and tumbler index were both improved. The reason for the benefits brought by CO can be described as adding CO improved the reducing atmosphere in sintering process. Moreover, with the recirculation of CO in flue gas, the combustion reaction (CO+ O2→CO2) occurred, which would enhance the maximum sintering temperature and prolonged the high-temperature duration time [33]. This contributed to improving the formation of liquid phase in granules. Finally, CO addition reduced the oxygen potential in sintering flue gas and thus prevented the reduced iron in the upper layer from being oxidized again.
3.4 Microstructural observation of sinter
The microstructures of the sinter were characterized by optical microscope analysis to explain the reasons for the improvement of sinter strength by PSPfsg-FGR. Figure 10 displays the microstructure of product sinter of TSP and PSPfsg-FGR.
As can be seen from Figures 11(a) and (b), irregular macropores with thin wall and wide cracks existed in conventional sinter, which were extremely detrimental to the sinter quality. It can be clearly observed in Figure 11(c) that in the sinter of TSP there was a significant amount of needle-like calcium ferrite, which was the most important bonding phase in sinter. In the meantime, magnetite was also observed with massive formation of silicates (Figure 11(d)). For PSPfsg-FGR (Figures 11(e) and (f)), the microstructure of pre-reduced sinter was transformed into small thick-wall pores and narrow cracks, substantially contributing to improving sinter strength. Furthermore, it also can be found that pre-reduced sinter (Figure 11(e)) had higher porosity than traditional sinter (Figure 11(a)). Therefore, pre-reduced sinter used as raw materials for blast furnace can avoid the problem of low temperature reduction pulverization in the upper part of blast furnace [34]. From Figures 11(g) and (h), it can be seen that pre-reduced sinter mainly consisted of magnetite (Fe3O4) and wustite (FexO). Small quantities of liquid phases i.e., silicate phases were formed in the gaps of magnetite and wustite. As reported in Ref. [35], in sinter product, the strength of silicate glass phase was poor. The formation of silicate glass phase should be minimized in the structure of sinter to obtain high sinter strength. There was a small quantity of silicate glass phase in pre-reduced sinter and no agglomeration of glass phase occurred. This may be another important reason for the higher strength of pre-reduced sinter.

As displayed in Figures 11(i)-(l), the iron in the pre-reduced sinter mainly existed in the form of wustite, which were mingled with some hematite and metallic iron. It can be seen from Figure 11(i) that the grains of wustite connected mutually, and a small amount of banded metallic iron existed at the junction of the grains. The silicate liquid phase filled in the gap between granular minerals and acted as bonding phase. Figures 11(j) and (k) displayed that the content of hematite in pre-reduced sinter was low, and the grains of hematite were extremely tiny and scattered around the pore of sinter. As can be seen from Figures 11(j), (k) and (l), metallic iron massively formed in the area with thin-walled and densely populated tiny pores. Meanwhile, thick band-like metallic iron phase was also found at the edge of macropores (Figure 11(l)). Furthermore, metallic iron with limited formation amount was also found at the edge of sinter as demonstrated in Figure 11(k). Due to the secondary oxidation of metallic iron distributed at the edge of the sinter, the metallic iron near the pores had a better effect of reduction atmosphere. The CO content in PSPfsg was higher than that in TSP caused by higher coke dosage. When CO passing through the pores and sinter surface, the wustite in the pores or sinter surface could be reduced to metallic iron from the outside to the inside. Therefore, metallic iron in the pre-reduced sinter was mainly formed by the indirect reduction of reducing gas CO and wustite.
3.5 PSPfsg-FGR practical implication of CO2 emission reduction and alkalis removal
In accordance with the benefits of FGR to PSPfsg, the authors believed that it is promising to achieve the industrial scale of this approach. Figure 12 shows the schematic diagram of PSPfsg-FGR in practical sinter machine. As indicated in Figure 12, due to the relatively high K and Na contents, the flue gas coming from the wind boxes of sintering tail was discharged into the air after dedusting and sulfur removal. While the flue gas emitted from the rest wind boxes had low K and Na contents, which was firstly purified with the help of an electrostatic precipitator and then recycled into the bed surface.
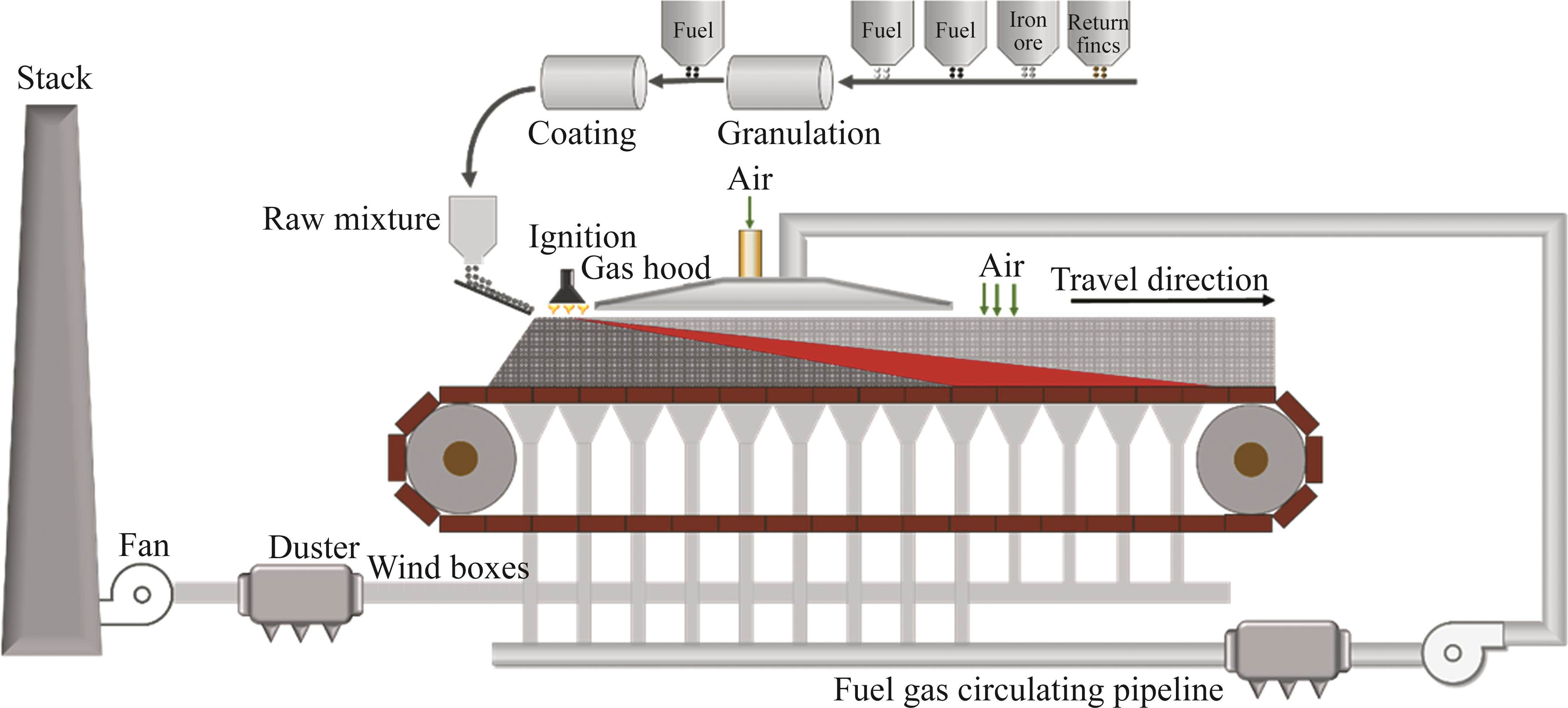
Figure 13 shows the process flow diagrams of traditional ironmaking process and the proposed ironmaking process, i.e., PSPfsg-FGR—blast furnace smelting. Conventionally, reduction of iron ore was mainly implemented by gas reduction in the blast furnace. Thus, reduction behavior in the blast furnace was restricted by gas reduction equilibrium. In addition, traditional sintering process can not realize the efficient removal of alkalis. Most of alkalis still remained in the sinter and entered into blast furnace eventually, which was detrimental to the operation of blast furnace. In the proposed ironmaking process, partial reduction of iron ore was achieved in the sintering process through the addition of extra coke breeze. With this method, direct reduction (solid reduction) by coke breeze also existed, which enabled reduction free from equilibrium restrictions of gas reaction. Moreover, the generated flue gas containing high content of CO was also a heat source, whose circulating could complete the process with a smaller amount of carbon. Using pre-reduced sinter obtained by PSPfsg-FGR as blast furnace burden can not only save energy in the traditional blast furnace process but also reduce the total energy consumption and the total CO2 emission in the entire iron making process. Furthermore, 74.11 wt% K and 32.92 wt% Na were removed and went into the dust, which realized the open circuit of harmful alkali elements in the ironmaking system and alleviated the harm of them to blast furnace.

In our study, a pre-reduction sintering process with flue gas recirculation (PSPfsg-FGR) was developed. It can be found that the proposed process possesses obvious some advantages. First, the developed process was simple and can be operated without changing the original sintering process and equipment. Then, the PSPfsg-FGR can not only mitigate alkalis harm to the blast furnace but also decrease the flue gas emission in the whole ironmaking process. Last, the flue gas recirculation was adopted to further increase the removal rate of alkali metals and significantly reduce CO2 emissions. These indicate that PSPfsg-FGR has great industrial application prospect.
4 Conclusions
In this study, a pre-reduction sintering process with flue gas recirculation (PSPfsg-FGR) was used to remove alkali metals from the raw mixture as well as reduce the flue gas emission from the whole iron making process. The main findings were summarized as follows.
1) For the traditional sintering process under the conditions of 8% moisture content and 5.5% coke dosage, the sinter indexes of yield, sintering speed, productivity and tumbler index were separately 68.68%, 21.61 mm/min, 1.53 t/(m2·h) and 65.70%, and the K and Na removal ratios were respectively 15.63% and 21.36%. The alkali content (K2O+Na2O) of sinter product reached 0.29%, which was higher than its threshold of 0.2% in Chinese industry standard.
2) Removal of alkali metals (K and Na) from the sinter through PSP is feasible, and the PSP has better conditions for the reduction of Fe3O4 to FeO and even can obtain the direct reduction iron after theoretical analysis.
3) PSP with fuel segregated granulation can effectively remove K and Na, and improve sintering quality. For 12% coke breeze dosage and 60% out-proportioning ratio of coke breeze, the yield and tumbler index were respectively 74.40% and 68.69%, while the productivity decreased to
1.18 t/(m2·h). The removal ratios of K and Na were 58.02% and 30.68%, respectively. The alkali content (K2O+Na2O) was 0.19%, which was lower than the threshold.
4) The NOx and SO2 emissions of PSPfsg were reduced and CO content was increased in comparison with TSP. The microstructures of pre-reduced sinter indicated that the main iron mineral phases were wustite, magnetite and a small amount of metallic iron. There was trace silicate glass phase in pre-reduced sinter.
5) The recirculation of simulated CO-containing flue gas further improved K and Na removal to 74.11% and 32.92%, respectively, and the metallization ratio rose to 5.86%. Meanwhile, it can achieve the reduction of total energy consumption and total CO2 emission in the entire iron making process. The technique provided a promising approach to remove alkali metal elements, save energy and reducing flue gas emission from the whole ironmaking flow.
A bottom-up analysis of China’s iron and steel industrial energy consumption and CO2 emissions
[J]. Applied Energy, 2014, 136: 1174-1183. DOI: 10.1016/j.apenergy.2014.06.002.Integrated assessment of exergy, energy and carbon dioxide emissions in an iron and steel industrial network
[J]. Applied Energy, 2016, 183: 430-444. DOI: 10.1016/j.apenergy.2016. 08.192.Estimates of the potential for energy conservation and CO2 emissions mitigation based on Asian-Pacific integrated model (AIM): The case of the iron and steel industry in China
[J]. Journal of Cleaner Production, 2014, 65: 120-130. DOI: 10.1016/j.jclepro.2013.09.008.Typical case of carbon capture and utilization in Chinese iron and steel enterprises: CO2 emission analysis
[J]. Journal of Cleaner Production, 2022, 363: 132528. DOI: 10.1016/j.jclepro.2022.132528.Discussion on the calculation method of CO2 emission in iron and steel enterprises
[C]//Reduction of CO2 emissions by use of pre-reduced iron ore as sinter raw material
[J]. ISIJ International, 2013, 53(9): 1625-1632. DOI: 10.2355/isijinternational.53.1625.Optimization of granulation and sintering performance of high-ratio magnet concentrate
[J]. Sintering and Pelletizing, 2024, 49(1): 56-64, 112. DOI: 10.13403/j.sjqt.2024.01.008.(in Chinese)Behavior of alkali metal removal and enrichment in particles during sintering process
[J]. Journal of Central South University (Science and Technology), 2017, 48(11): 2843-2850. DOI: 10.11817/j.issn.1672-7207.2017.11.001. (in Chinese)The use of thermodynamic modeling to examine alkali recirculation in the iron blast furnace
[J]. High Temperature Materials and Processes, 2012, 31(4, 5): 657-665. DOI: 10.1515/htmp-2012-0103.Process optimization and kinetics of titanium leaching from mechanically activated titanium-bearing blast furnace slag
[J]. Journal of Sustainable Metallurgy, 2023, 9(1): 230-239. DOI: 10.1007/s40831-022-00640-7.Enhancing the removal of sodium and potassium of sinter by CO-containing flue gas circulation sintering process
[C]//Damages of detrimental elements to blast furnaces of PZH steel
[J]. Iron & Steel, 2009, 44(4): 96-99. DOI: 10.3321/j.issn: 0449-749X.2009.04.022. (in Chinese)A study on hazard mitigation of alkali metals in blast furnace and alkaline drainage from slag
[J]. Chemical Enterprise Management, 2024(7): 62-65. DOI: 10.19900/j.cnki.ISSN1008-4800.2024.07.018. (in Chinese)Reaction and distribution of alkali metals in blast furnace
[J]. Journal of Iron and Steel Research, 2006, 18(6): 6-10. DOI: 10.3321/j.issn: 1001-0963.2006.06.002. (in Chinese)Influence of dioxin reduction on chemical composition of sintering exhaust gas with adding urea
[J]. Journal of Central South University, 2012, 19(5): 1359-1363. DOI: 10.1007/s11771-012-1150-y.Study on flow direction and balance of alkali-metals in sintering process
[J]. Metal Materials and Metallurgy Engineering, 2017, 45(2): 7-12. DOI: 10.16793/j.cnki.2095-5014.2017.02.002. (in Chinese)Influence of alkali metal on the process of sintering
[J]. Journal of Inner Mongolia University of Science and Technology, 2010, 29(2): 108-111. DOI: 10.3969/j.issn.2095-2295.2010.02.003. (in Chinese)Reducing Na2O and K2O contents in Baotou iron concentrate by flotation
[J]. Metal Mine, 2006(2): 31-35. DOI: 10.3321/j.issn: 1001-1250.2006.02.009. (in Chinese)Existing state of potassium chloride in agglomerated sintering dust and its water leaching kinetics
[J]. Transactions of Nonferrous Metals Society of China, 2011, 21(8): 1847-1854. DOI: 10. 1016/S1003-6326(11)60940-0.Analysis on behavior mechanism of K2O and Na2O in sintering
[J]. Science & Technology of Baotou Steel, 2012, 38(6): 33-36, 49. DOI: 10.3969/j.issn.1009-5438.2012. 06.011.Co-benefits of CO2 emission reduction and sintering performance improvement of limonitic laterite via hot exhaust-gas recirculation sintering
[J]. Powder Technology, 2020, 373: 727-740. DOI: 10.1016/j.powtec.2020.07.018.Behavior of alkalis in blast furnaces
[J]. Metallurgist, 2016, 59(9): 761-765. DOI: 10.1007/s11015-016-0171-4.Phase evolution of tin, iron and calcium oxides roasted in a simulative sintering atmosphere
[J]. Powder Technology, 2017, 311: 303-312. DOI: 10.1016/j.powtec.2017.01.053.Research of rational vertical sintering speed
[J]. Journal of Central South University (Science and Technology), 2007, 38(2): 245-250. DOI: 10.3969/j.issn.1672-7207.2007.02.012.Effect of coke breeze distribution on coke combustion rate of the quasi-particle
[J]. ISIJ International, 2015, 55(12): 2550-2555. DOI: 10.2355/isijinternational.isijint-2015-089.Insight into the high proportion application of biomass fuel in iron ore sintering through CO-containing flue gas recirculation
[J]. Journal of Cleaner Production, 2019, 232: 1335-1347. DOI: 10.1016/j.jclepro.2019.06.006.Research status of pre-reduction sintering technology and development on the new technology
[J]. Sintering and Pelletizing, 2017, 42(6): 22-26, 38. DOI: 10.13403/j.sjqt.2017.06.069.(in Chinese)ZHONG Qian, JIANG Wen-zheng, GAO Wei, LI Qian, YANG Yong-bin and JIANG Tao declare that they have no conflict of interest.
ZHONG Qiang, JIANG Wen-zheng, GAO Wei, LI Qian, YANG Yong-bin, JIANG Tao. Pre-reduction sintering process with flue gas recirculation for reduction alkalis harm and flue gas emission [J]. Journal of Central South University, 2025, 32(1): 106-121. DOI: https://doi.org/10.1007/s11771-024-5770-9.
钟强,姜文政,高伟等.预还原烧结烟气循环工艺降低碱危害及烟气排放[J].中南大学学报(英文版),2025,32(1):106-121.