1 Introduction
Arsenopyrite is an important gold-bearing sulfide in arsenic-containing refractory gold ores [1]. The fine disseminated gold is often encapsulated in arsenopyrite matrix. It is necessary to pretreat these ores before cyanidation to improve gold recovery [2]. However, there are potential environmental threats associated with conventional pretreatment methods including oxidation roasting [3, 4], pressure oxidation [5], and chemical oxidation [6] for arsenic-containing refractory gold ores [7]. The release and the harmless treatment of arsenic have become a challenge for industrial production. Bio-oxidation technology has garnered considerable attention for treating these gold ores [8], as the arsenic in arsenopyrite becomes soluble ions during bio-oxidation and can subsequently be solidified into a stable precipitate through neutralization [9, 10]. Nevertheless, large-scale application is limited by slow dissolution kinetics. This can be attributed to the inhibitory effect of passivation film [11, 12] and the low microbial activity [13].
It is necessary to improve the microbial activity during arsenopyrite bio-oxidation. Studies show that supplementing ferric iron or adding pyrolusite could improve the microbial activity [14, 15]. Similarly, optimizing the reactor to reduce shearing and frictional interactions between mineral particles and microorganisms could also yield similar results [16]. Eliminating or attenuating the effect of passive layer is the key to increasing the bio-oxidation rate of arsenopyrite. Studies reveal that the addition of Cu2+ and Ag+ contributed to building Cu2+/(Cu2S/CuS) and Ag+/Ag2S catalytic cycles to eliminate the passive film on the arsenopyrite surface [17, 18]. In addition, adding FeCl3 as ferric iron supplement to the system [19] or using a mixed culture of iron-oxidizing and sulfur-oxidizing microorganisms [20] also contributed to the removal of passive film. What is more, ZHANG et al [21] pointed out that the addition of humic acid could not only promote the elimination of S0, but also facilitate the arsenic immobilization. Notably, an innovative chemical-biological two-stage oxidation process had been proposed for arsenic-bearing high-sulfur refractory gold concentrates. This process could improve the bio-oxidation efficiency through a short, environmentally friendly chemical oxidation of ferric iron, without extra addition of mineral or metal ions [22, 23]. In previous study, we modified the two-stage oxidation process by using the bio-oxidation waste solution for chemical oxidation instead of the ferric iron solution [24]. Interestingly, the study showed that chemical oxidation of bio-oxidation waste solution (BOS) was more beneficial than that of pure ferric iron solution (Fe2(SO4)3) to improve microbial activity and increase the bio-oxidation efficiency.
In this study, the effects of changes in particle size, surface morphology, surface structure of arsenopyrite in chemical oxidation on the microbial growth and adsorption, microbial community structure in the bio-oxidation were systematically discussed, aiming to reveal the mechanism by which the dissolution kinetics of arsenopyrite is enhanced in the two-stage oxidation process.
2 Materials and methods
2.1 Microorganism
The mixed culture HQ0211 used in this study was provided by Bio-metallurgical Laboratory, Northeastern University, China. For bio-oxidation experiments, 10 mL of the microbial suspension was separately inoculated into 18 Erlenmeyer flasks of 500 mL, each of them containing 90 mL of 9K medium without ferrous iron. The initial microbial concentration was 2.8×107 cells/mL.
2.2 Concentrate sample
The arsenopyrite sample used in this study was obtained from Guilin Qinglang Mining Company, China, with 90% particles <74 μm. The sample contained 50.9% As, 27.4% Fe, and 20.7% S. In addition, the X-ray diffraction result indicated that the arsenopyrite was the main mineral phase, as shown in Figure S1.
2.3 Chemical-biological oxidation experiments
The chemical oxidation of arsenopyrite was performed in both ferric sulfate solution (FSS) and BOS systems. The BOS was prepared in laboratory bio-oxidation experiment by oxidizing the gold concentrate. The microbial concentration in BOS was 3.6×109 cells/mL. The FSS was prepared by ferric sulfate making it have the same Fe3+ concentration and initial pH as the BOS. The chemical parameters of the two solutions are shown in Table 1. In the experiment, arsenopyrite was added to a stirred reactor containing 3 L of solution, making the pulp density of 2.5% (w/V). The reaction temperature and stirring speed were set to 80°C and 400 r/min, respectively. After 4 h, the oxidized residues were obtained by solid-liquid separation operation of the pulp.
| Process system | ORP/mV | pH | C(As)/(g·L-1) | TFe/(g·L-1) | C(Fe2+)/(g·L-1) |
|---|---|---|---|---|---|
| FSS | 677±6 | 0.92±0.01 | 0.0±0.0 | 16.1±0.1 | 0.0±0.0 |
| BOS | 715±4 | 1.02±0.01 | 4.2±0.0 | 16.2±0.1 | 0.0±0.0 |
The bio-oxidation experiments were conducted for the two chemical oxidation residues and untreated arsenopyrite. Specifically, 2.5 g of the solid sample was added to a 500 mL Erlenmeyer flask containing the microbial inoculum, resulting in a pulp density of 2.5%. The pH was adjusted to 1.60±0.02. The bio-oxidation experiment of each sample was carried out simultaneously in six such flasks on a rotary shaker, with the temperature set to 42 ℃ and the rotating speed set to 180 r/min. Every three days, one flask was randomly selected from each experimental group and subjected to solid-liquid separation. All experiments were conducted in duplicate.
2.4 Calculation methods
The extraction rates of As, Fe and S elements in solid samples were calculated by Eq. (1).
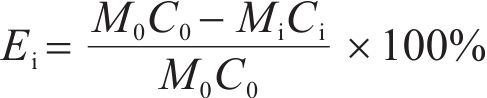
where the C0 and Ci are the contents of As, Fe and S elements in the initial material and oxidized residues, respectively, and M0 and Mi are the masses of the initial material and oxidized residues.
3 Results and discussion
3.1 High-temperature chemical oxidation experiments
3.1.1 The results of chemical oxidation
Chemical oxidation was an oxidation process with Fe3+ as the primary oxidant [22]. The oxidation behaviors (ORP, pH, C(Fe2+) and C(Fe2+)) of arsenopyrite were exhibited in Figure S2.
As shown in Table 2, after 4 h, the extraction rates of As, Fe, and S elements were (19.8±1.2)%, (21.8±0.9)%, and (3.4±0.8)%, respectively, after oxidation by the FSS system, whereas they were (14.2±2.0)%, (15.6±2.4)% and (2.6±1.8)% after oxidation by the BOS system. The oxidation capacity of the BOS system was lower than that of the FSS system, despite having the same initial Fe3+ concentration in both systems.
| Process system | Weight loss/% | Content in oxidized residue/wt% | Extraction rate/% | ||||
|---|---|---|---|---|---|---|---|
| As | Fe | S | As | Fe | S | ||
| FSS | 16.0±0.4 | 48.6±0.7 | 25.5±0.3 | 23.8±0.2 | 19.8±1.2 | 21.8±0.9 | 3.4±0.8 |
| BOS | 7.9±0.9 | 47.4±1.1 | 25.1±0.7 | 21.9±0.4 | 14.2±2.0 | 15.6±2.4 | 2.6±1.8 |
3.1.2 The physical changes for minerals after chemical oxidation
The physical changes observed in arsenopyrite after chemical oxidation could be summarized in two main aspects. Firstly, the mineral surface morphology was significantly altered, accompanied by the formation of numerous microcracks, which was evident in the SEM images presented in Figure S3. Arsenopyrite, being a mineral with moderate cleavage, exhibited corrosion behavior primarily along the cleavage plane where the bonding was relatively weak [25], resulting in the formation of microcracks on the mineral surface. Notably, microorganisms tend to selectively attach to mineral surfaces with defects and low crystallinity [26]. Therefore, the altered surface morphology may provide favorable conditions for the microbial attachment during bio-oxidation.
Secondly, chemical oxidation leads to a reduction in the particle size and an increase in the specific surface area of arsenopyrite, as depicted in Figure S4. The particle size reduction was more pronounced in the FSS system compared to the BOS system. The decrease in particle size and the corresponding increase in specific surface area created favorable conditions for enhanced bio-oxidation processes.
3.1.3 The chemical changes for minerals after chemical oxidation
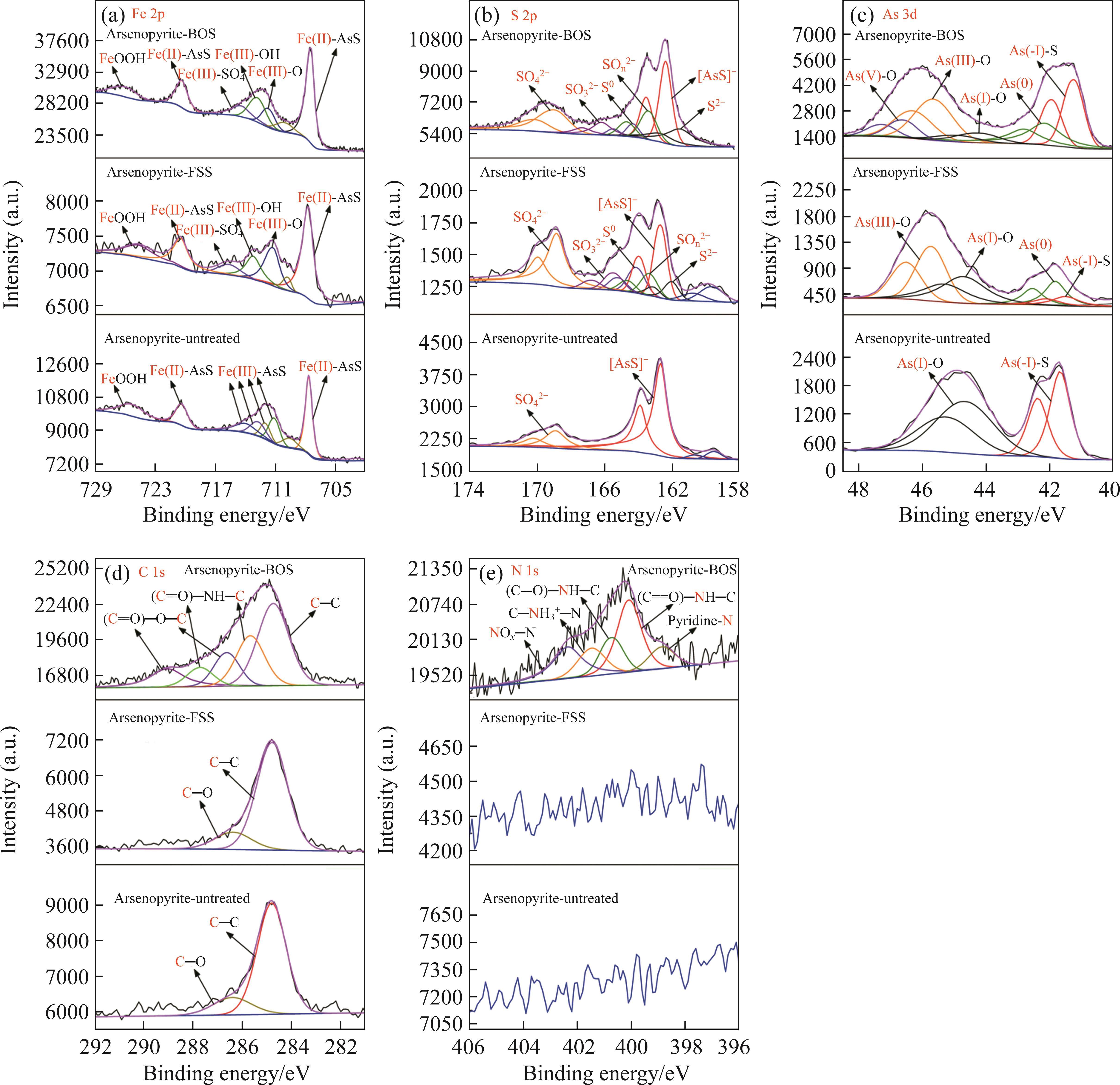
Raman spectroscopy was employed to analyze the changes of arsenopyrite surface during chemical oxidation, and the results are depicted in Figure 1. The characteristic peaks of the initial arsenopyrite surface were observed at 203, 222, 287 and 404 cm-1 [27]. After oxidation, the emergence of peaks at 152, 219 and 472 cm-1 indicated the presence of 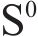
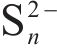
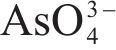
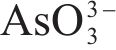
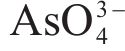
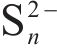
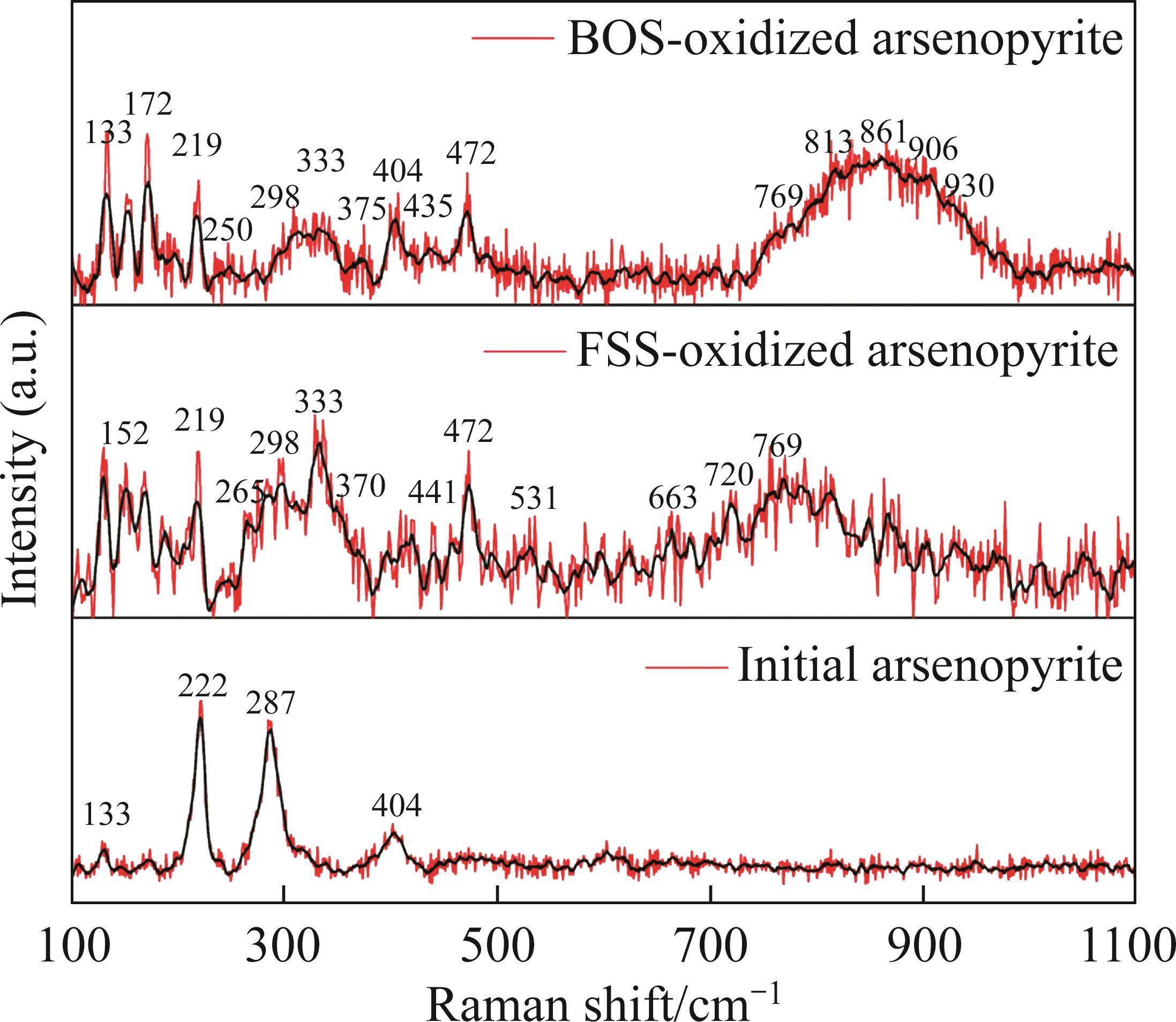
Next, the change of chemical structure of arsenopyrite surface was analyzed by XPS, the fitted results are shown in Figure 2. Table 3 displays the XPS analysis data for arsenopyrite Fe, S, As. It was found that the chemical structure of arsenopyrite surface was disrupted during chemical oxidation, accompanied by the formation and accumulation of oxidation products. The oxidation steps for As and S elements of arsenopyrite could be established: for S, 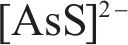
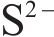
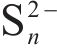
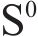
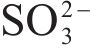
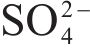
| Binding energy/eV | Designation | Reference | |
|---|---|---|---|
| Fe 2p | 707.65, 709.58,720.35 | Fe(II)-AsS | [33] |
| 711.11, 711.99, 712.73, 714.00 | Fe(III)-AsS | ||
| 711.21, 713.19, 715.64 | FeOOH Fe(III)-OH Fe(III)-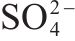 | [34, 35] | |
| S 2p | 162.7 | 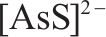 | [36] |
| 168.91 | 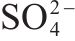 | ||
| 161.62±0.05 | 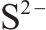 | [37, 38] | |
| 163.40±0.02 | 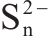 | ||
| 164.22±0.04 | 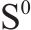 | ||
| 165.82±0.18 | 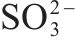 | ||
| As 3d | 41.67 | As(-I)-S | [39] |
| 44.66 | As(I)-O | ||
| 41.81 | As(0) | [34, 40] | |
| 45.73 | As(III)-O | ||
| 46.59 | As(V)-O | ||
| C 1s | 284.80, 286.40 | C—C, C—O | [41] |
| 285.67, 287.71 | (C=O)—NH—C | [42] | |
| 286.95, 289.03 | (C=O)—O—C | [43] | |
| N 1s | 398.86 | Pyridine | [44] |
| 400.07, 400.71 | (C=O)—NH—C | [45, 46] | |
| 401.47 | C— | [42] | |
| 402.29 | NOx | [44] | |
In addition, as shown in Table 4, the nitrogen content of arsenopyrite sample increased from initial (0.07±0.02)% to (1.08±0.11)% after BOS oxidation, but did not change noticeably after FSS oxidation. This was associated with the presence of microorganisms and organic matter in the BOS system. Meanwhile, Table 3 indicates that the nitrogenous substances predominantly exist in the form of organic N, including pyridine-N, protein-N, protonated amine-N, with a minor presence of inorganic nitrogen oxides-NOx-N.
| Sample | Content/% |
|---|---|
| Initial arsenopyrite | 0.07±0.02 |
| FSS-oxidized arsenopyrite | 0.06±0.02 |
| BOS-oxidized arsenopyrite | 1.08±0.11 |
3.2 Bio-oxidation experiments
3.2.1 Bio-oxidation of arsenopyrite
The bio-oxidation of arsenopyrite could be characterized by reactions (1)-(3), and the changes in relevant parameters are depicted in Figure 3.
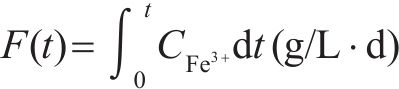
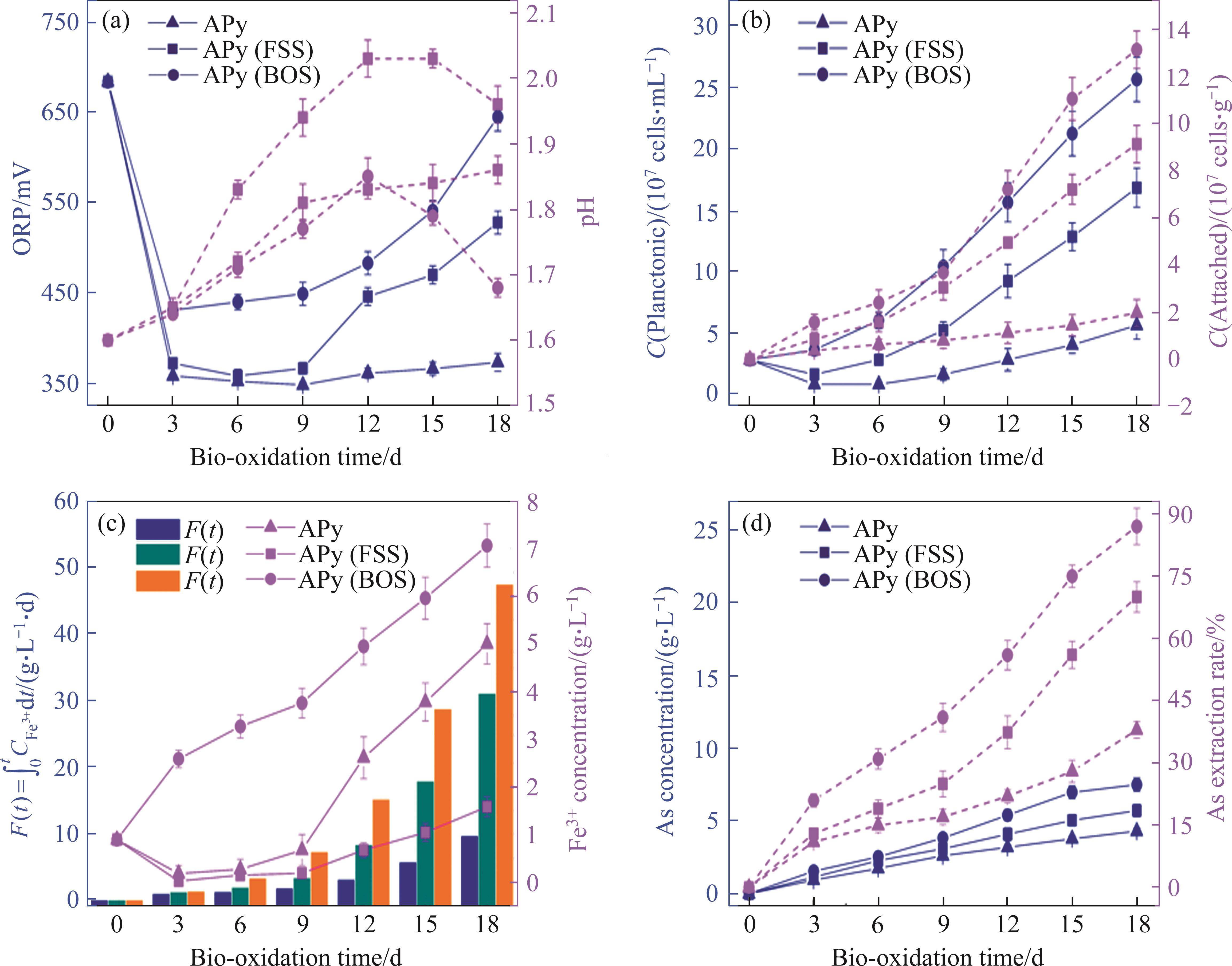
It was observed that the microbial growth in both FSS-oxidized arsenopyrite and BOS-oxidized arsenopyrite systems exhibited notable acceleration in comparison to the untreated arsenopyrite system, resulting in a rapid increase in Fe3+ concentration and ORP in systems. The oxidation speed of arsenopyrite was found to be significantly increased in these two systems. This could be attributed to the structural disruption of mineral surface and the reduction in particle size induced by chemical oxidation. The alterations made arsenopyrite more susceptible to oxidation during bio-oxidation. Concurrently, the augmented release of Fe2+ also created a favorable environment for microbial growth. Figure 3(b) demonstrates a greater number of attached microorganisms on the surfaces of both chemically oxidized residues. This phenomenon could be attributed to the disruption of surface morphology, which resulted in an increased number of favorable sites for microbial attachment.

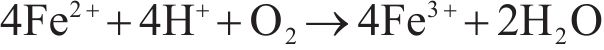
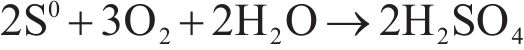
Table 5 presents the oxidation results. After 18 d, the two-stage oxidation process applying BOS extracted (88.8±2.0)%, (86.7±1.3)% and (74.7±3.0)% of As, Fe, and S elements, respectively. These values represented significant increasing amounts of (50.8±3.4)%, (47.1±2.7)% and (46.0±0.7)%, respectively, compared to the one-stage bio-oxidation process. The oxidation efficiency of arsenopyrite was significantly improved in two-stage oxidation process applying BOS.
| Oxidation process | Process system | Weight loss/% | Content in oxidized residue/% | Extraction rate/% | ||||
|---|---|---|---|---|---|---|---|---|
| As | Fe | S | As | Fe | S | |||
| One-stage oxidation | Arsenopyrite | 22.3±2.1 | 40.6±0.9 | 21.3±0.5 | 19.0±0.6 | 38.0±1.4 | 39.6±1.4 | 28.7±2.3 |
| FSS residue | 56.1±3.2 | 33.1±2.9 | 16.9±1.1 | 23.3±1.3 | 70.1±2.6 | 70.9±1.9 | 57.0±2.4 | |
| BOS residue | 70.5±3.4 | 21.0±3.7 | 13.4±1.3 | 19.3±2.3 | 86.9±2.3 | 84.3±1.5 | 74.0±3.1 | |
| Two-stage oxidation | Total extraction rate (FSS+HQ0211) | 76.0±2.1 | 77.3±1.5 | 58.5±2.3 | ||||
| Total extraction rate (BOS+HQ0211) | 88.8±2.0 | 86.7±1.3 | 74.7±3.0 | |||||
3.2.2 Microbial community structure and diversity
The analysis results of microbial community structure and diversity are shown in Figure 4. Initially, the microbial community was dominated by Leptospirillum spp. accounting for 86.0%, along with Sulfobacillus spp. comprising 13.8%, and small proportions of Ferroplasma spp. and other genera.
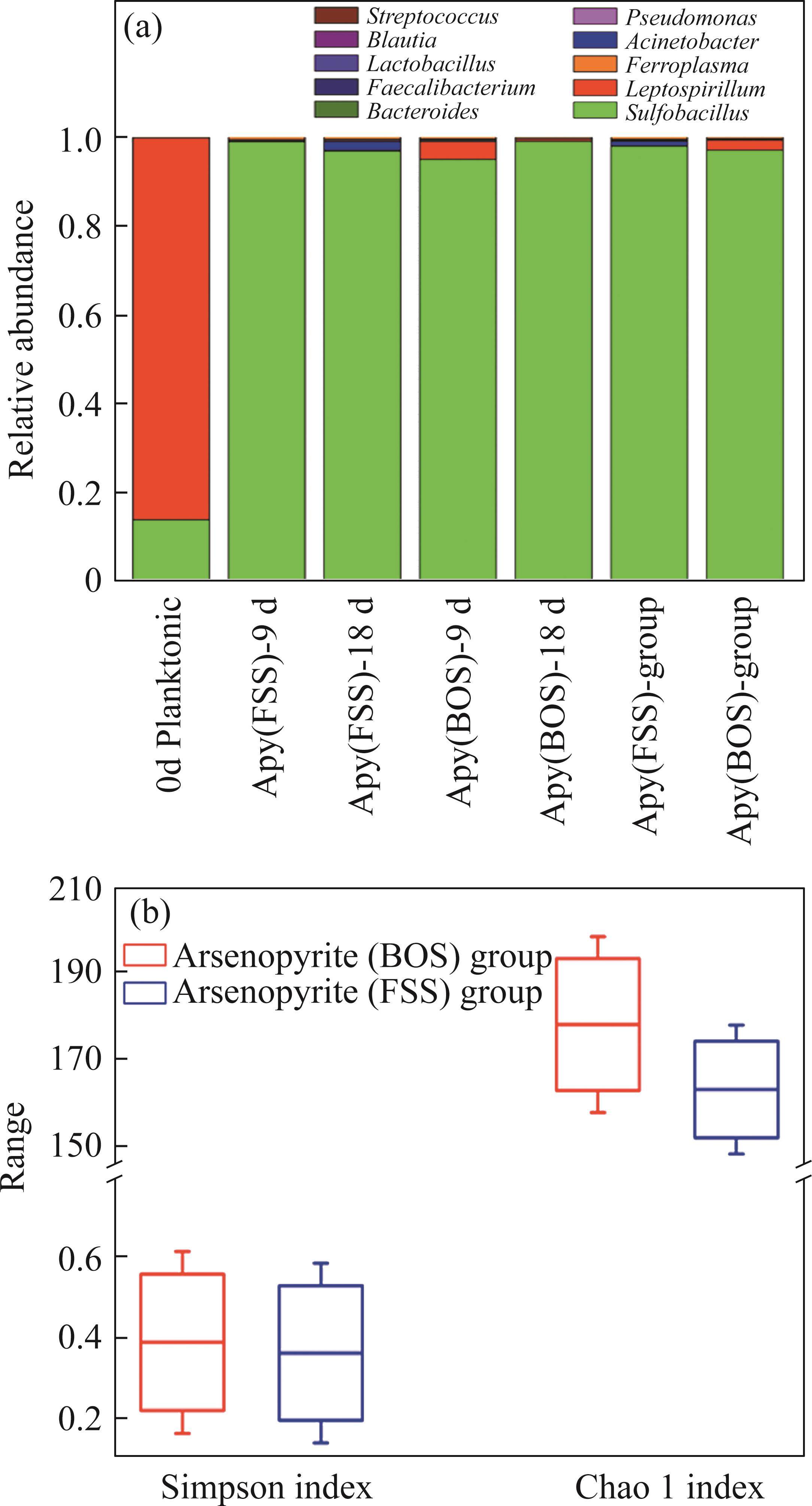
During bio-oxidation, the Sulfobacillus spp. replaced Leptospirillum spp. as the dominant microorganisms. Its total proportion in the microbial community exceeded 90% in both systems. The total proportion of Leptospirillum spp. was 2.32% in the BOS-oxidized arsenopyrite system, which was significantly higher than that of 0.14% in the FSS-oxidized arsenopyrite system. Previous analyses revealed that the S0 and sulfur compounds had been generated on the mineral surface after chemical oxidation. Moreover, these substances were also produced during bio-oxidation, along with the release of Fe2+. The Sulfobacillus spp. have the ability not only to oxidize Fe2+ but also to metabolize S0 and sulfur-containing compounds [47], whereas Leptospirillum spp. can only oxidize Fe2+ [48]. Consequently, the environment was more conducive to Sulfobacillus spp. growth. The proportion of Leptospirillum spp. in BOS-oxidized arsenopyrite system was higher than that in the FSS-oxidized arsenopyrite system, which could be attributed to the effect of nitrogenous substances on microbial growth. Additionally, Figure 4(b) reveals that both the Chao 1 and Simpson index of BOS-oxidized arsenopyrite system were higher than those of the FSS-oxidized arsenopyrite system. It is suggested that chemical oxidation of BOS also contributed to improving the Alpha diversity of microbial community.
3.2.3 Kinetic analysis
Previous studies commonly employed the shrinking core model to describe the dissolution process of arsenopyrite [13, 49]. This model combines interfacial chemical reaction control (Eq. (2)) and diffusion control (Eq. (3)) [50, 51]. Initially, the dissolution of arsenopyrite was fitted using these two conventional control equations. However, neither of the conventional kinetic equations could accurately predict the actual oxidation data (Figure 5(a)). In fact, the bio-oxidation of sulfides is an indirect oxidation process where Fe3+ acts as the oxidant [52, 53]. The Fe3+ concentration would vary during bio-oxidation. However, the fitting of the conventional kinetic equations to this process had been carried out under the assumption of a constant Fe3+concentration [54], which was the main reason for the poor fitting results.
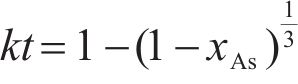
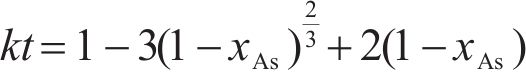
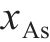
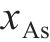
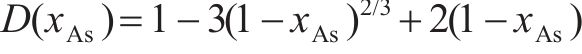
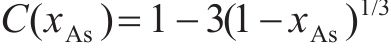
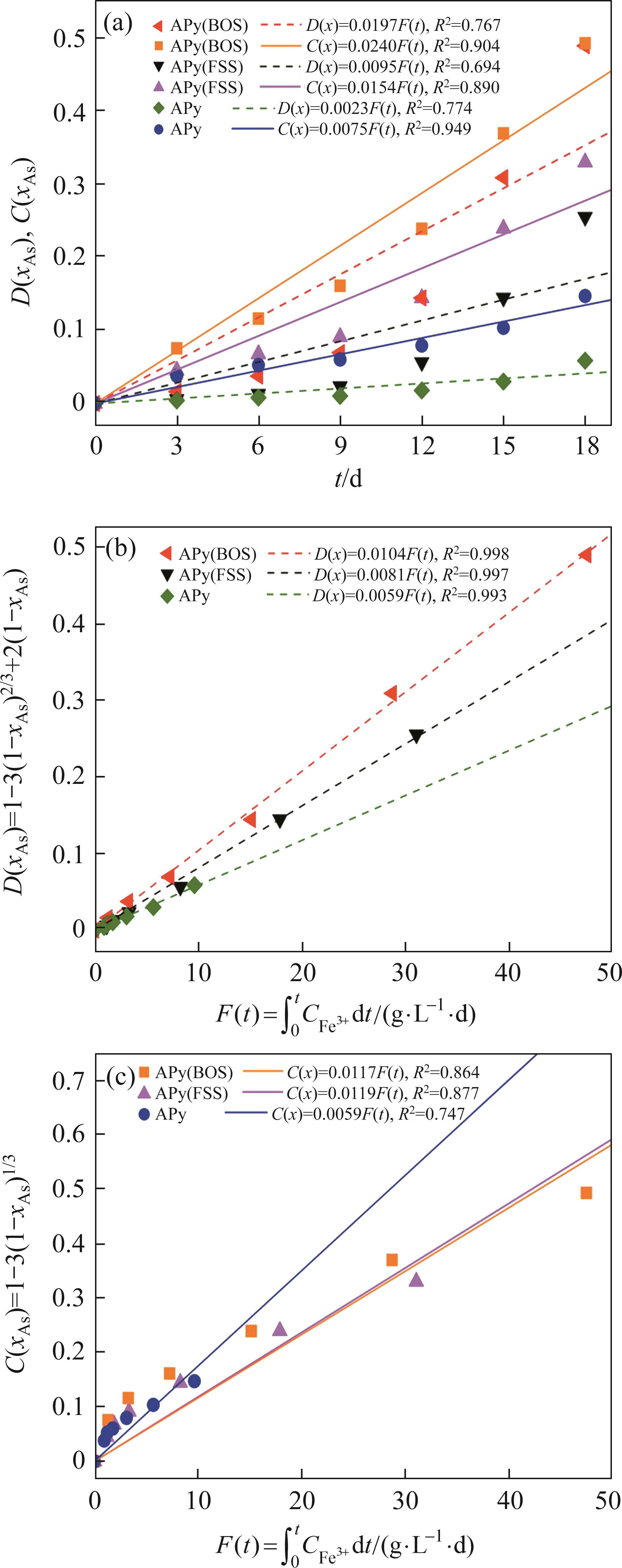
where k is the reaction rate constant; t is the bio-oxidation time; 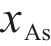
To consider the variation of Fe3+ concentration, the time t in the conventional kinetic equations was replaced with the Fe3+ concentration integral for time, as shown in Eq. (4) and Eq. (5). The value of F(t) during the bio-oxidation was graphically determined by OriginPro 9.0 software. Next, the two revised kinetic equations were used to fit the arsenopyrite dissolution process, as shown in Figure 5. The results reveled that the revised equations provided a more accurate description and prediction of the dissolution process. The R-squared values of the revised diffusion control for three samples were all significantly higher than those of the revised chemical reaction control. Moreover, the SEM-EDS analysis results also evidenced that the passive film consisting of S0 and arsenate was formed on the bio-oxidized residue surface (Figure S5). Therefore, it could be determined that the dissolution rate of arsenopyrite was mainly controlled by the diffusion step during bio-oxidation. Notably, the passive film on the FSS-oxidized arsenopyrite surface appeared more compact compared to that on the BOS-oxidized arsenopyrite surface. The rise level of pH in former system was also higher than that in latter system. These suggested that the microorganisms in the BOS-oxidized arsenopyrite system were more efficient in decomposing elemental sulfur in the passivated film, which was significant for accelerating the dissolution of arsenopyrite.
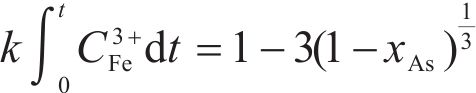
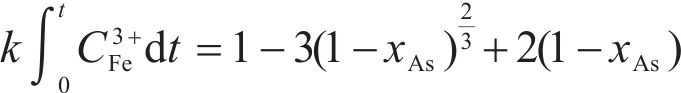
The values of reaction rate constant k for untreated arsenopyrite, FSS-oxidized arsenopyrite and BOS-oxidized arsenopyrite were 0.0059, 0.0081 and 0.0104, respectively (Figure 5(b)). It was evident that the dissolution kinetics of arsenopyrite in bio-oxidation was enhanced after chemical oxidation. This was attributed to the following three main reasons. Firstly, chemical oxidation destroyed the chemical structure of arsenopyrite surface, making it more susceptible to dissolution. Secondly, chemical oxidation reduced arsenopyrite particle size while destroying mineral surface morphology. This change enhanced the accessibility of ions and created a favorable condition for microbial attachment. Lastly, chemical oxidation increased the release efficiency of substances available for microbial metabolism, and led to the generation of nitrogenous substances on the mineral surface. This improved microbial activity and microbial community structure. This improvement not only promoted the Fe3+ cycle, but also more importantly, enhanced the decomposition of elemental sulfur attached to the arsenopyrite surface, thereby reducing its inhibitory effect on ion diffusion during reaction process. For these reasons, the bio-oxidation efficiency of arsenopyrite in the two-stage oxidation process was significantly enhanced.
4 Conclusions
Applying bio-oxidation waste solution to two-stage oxidation process could significantly enhance the bio-oxidation efficiency of arsenopyrite. Studies showed that the dissolution rate of arsenopyrite in bio-oxidation was mainly controlled by the diffusion of ions through passivation layer composed of elemental sulfur and arsenate. The enhanced dissolution kinetics could be attributed to the following the three reasons:
1) Chemical oxidation contributed to destroying the surface chemical structure of arsenopyrite, making it more susceptible to dissolution during bio-oxidation;
2) Chemical oxidation reduced the particle size of arsenopyrite, enhancing the accessibility of ions. In addition, the disruption of mineral surface morphology also created a favorable condition for microbial attachment;
3) Chemical oxidation increased the release efficiency of substances available for microbial growth and resulted in the generation of nitrogenous substances (pyridine-N, protein-N, protonated amine-N, and NOx-N), thereby improving microbial activity and microbial community structure. These improvements not only effectively increased oxidant-Fe3+ concentration, but more importantly enhanced the metabolism of elemental sulfur by microorganisms thus reducing its inhibitory effect. It is essential to enhance the dissolution kinetics of arsenopyrite.
New insights about the bacterial oxidation of arsenopyrite: A mineralogical scope
[J]. Minerals Engineering, 2012, 39: 248-254. DOI: 10.1016/j.mineng.2012.06.012.Effect of mechanical activation on thiosulfate leaching of gold from complex sulfide concentrate
[J]. Transactions of Nonferrous Metals Society of China, 2011, 21(12): 2744-2751. DOI: 10.1016/S1003-6326(11)61118-7.Recovery of gold from sulfide refractory gold ore: Oxidation roasting pretreatment and gold extraction
[J]. Minerals Engineering, 2021, 164: 106822. DOI: 10.1016/j.mineng.2021.106822.Recovery of iron from pyrite cinder by suspension magnetization roasting-magnetic separation method: Process optimization and mechanism study
[J]. Separation and Purification Technology, 2024, 332: 125652. DOI: 10.1016/j.seppur. 2023.125652.Research on chlorination leaching of pressure-oxidized refractory gold concentrate
[J]. Hydrometallurgy, 2020, 194: 105325. DOI:10.1016/j.hydromet.2020.105325.Pretreatment of a refractory arsenopyritic gold ore using hydroxyl ion
[J]. Hydrometallurgy, 2015, 153: 106-113. DOI: 10.1016/j.hydromet.2015.02.013.Biooxidation as pre-treatment for a telluride-rich refractory gold concentrate
[J]. Minerals Engineering, 2000, 13(12): 1219-1229. DOI: 10.1016/S0892-6875(00)00106-0.Microbially-assisted dissolution of minerals and its use in the mining industry
[J]. Pure and Applied Chemistry, 2004, 76(4): 847-859. DOI: 10.1351/pac200476040847.Arsenic toxicity, health hazards and removal techniques from water: An overview
[J]. Desalination, 2007, 217(1-3): 139-166. DOI: 10.1016/j.desal.2007.01.015.Arsenic removal from water/wastewater using adsorbents: A critical review
[J]. Journal of Hazardous Materials, 2007, 142(1-2): 1-53. DOI: 10.1016/j.jhazmat.2007.01.006.Surface characterization of arsenopyrite during chemical and biological oxidation
[J]. Science of the Total Environment, 2018, 626: 349-356. DOI: 10.1016/j.scitotenv.2018.01.099.Comparative study of inorganic arsenic resistance of several strains of Acidithiobacillus thiooxidans and Acidithiobacillus ferrooxidans
[J]. Hydrometallurgy, 2009, 98(3, 4): 235-240. DOI: 10.1016/j.hydromet.2009.05.004.Formation process of the passivating products from arsenopyrite bioleaching by acidithiobacillus ferrooxidans in 9K culture medium
[J]. Metals, 2019, 9(12): 1320. DOI: 10.3390/met91 21320.Mechanism by which ferric iron promotes the bioleaching of arsenopyrite by the moderate thermophile Sulfobacillus thermosulfidooxidans
[J]. Process Biochemistry, 2019, 81: 11-21. DOI: 10.1016/j.procbio.2019.03.004.Enhancement of bio-oxidation of refractory arsenopyritic gold ore by adding pyrolusite in bioleaching system
[J]. Transactions of Nonferrous Metals Society of China, 2016, 26(9): 2479-2484. DOI: 10.1016/S1003-6326(16)64339-X.Arsenopyrite bioleaching by Acidithiobacillus ferrooxidans in a rotating-drum reactor
[J]. Minerals Engineering, 2012, 39: 19-22. DOI: 10.1016/j.mineng.2012.07.018.Role of Ag+ in the bioleaching of arsenopyrite by Acidithiobacillus ferrooxidans
[J]. Metals, 2020, 10(3): 403. DOI: 10.3390/met10030403.The catalytic effect of copper ion in the bioleaching of arsenopyrite by Acidithiobacillus ferrooxidans in 9K culture medium
[J]. Journal of Cleaner Production, 2020, 256: 120391. DOI: 10.1016/j.jclepro.2020.120391.Enhancement of arsenopyrite bioleaching by different Fe(III) compounds through changing composition and structure of passivation layer
[J]. Journal of Materials Research and Technology, 2020, 9(6): 12364-12377. DOI: 10.1016/j.jmrt. 2020.08.088.Bioleaching of arsenopyrite by mixed cultures of iron-oxidizing and sulfur-oxidizing microorganisms
[J]. Chemosphere, 2017, 185: 403-411. DOI: 10.1016/j.chemosphere.2017.07.037.Humic acid promotes arsenopyrite bio-oxidation and arsenic immobilization
[J]. Journal of Hazardous Materials, 2020, 384: 121359. DOI: 10.1016/j.jhazmat.2019.121359.Two-stage bacterial-chemical oxidation of refractory gold-bearing sulfidic concentrates
[J]. Hydrometallurgy, 2010, 101(1, 2): 28-34. DOI: 10.1016/j.hydromet.2009. 11.009.Bio-oxidation of a high-sulfur and high-arsenic refractory gold concentrate using a two-stage process
[J]. Minerals Engineering, 2018, 120: 94-101. DOI: 10.1016/j.mineng. 2018.02.013.Two-stage chemical-biological oxidation process for low-grade refractory gold concentrate with high arsenic and sulfur
[J]. Minerals Engineering, 2023, 191: 107976. DOI: 10.1016/j.mineng.2022.107976.Electronic and structural properties of bulk arsenopyrite and its cleavage surfaces-A DFT study
[J]. RSC Advances, 2015, 5(3): 2013-2023. DOI: 10.1039/C4RA13807D.Investigation of the attachment of Thiobacillus ferrooxidans to mineral sulfides using scanning electron microscopy analysis
[J]. Minerals Engineering, 2000, 13(6): 643-656. DOI: 10.1016/S0892-6875(00)00046-7.Assessment of biofilm changes and concentration-depth profiles during arsenopyrite oxidation by Acidithiobacillus thiooxidans
[J]. Environmental Science and Pollution Research, 2017, 24(24): 20082-20092. DOI: 10. 1007/s11356-017-9619-8.Influence of the sulfur species reactivity on biofilm conformation during pyrite colonization by Acidithiobacillus thiooxidans
[J]. Applied Microbiology and Biotechnology, 2012, 95(3): 799-809. DOI: 10.1007/s00253-011-3715-3.Raman spectroscopic study of cinnabar (HgS), realgar (As4S4), and orpiment (As2S3) at 298 and 77 K
[J]. Neues Jahrbuch Für Mineralogie-Monatshefte, 2002, 2002(10): 469-480. DOI: 10.1127/0028-3649/2002/2002-0469.Raman spectroscopic identification of arsenate minerals in situ at outcrops with handheld (532 nm, 785 nm) instruments
[J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2016, 154: 193-199. DOI:10.1016/j.saa.2015. 10.025.Raman spectroscopic study of the arsenite minerals leiteite ZnAs2O4, reinerite Zn3(AsO3)2 and cafarsite Ca5(Ti, Fe, Mn)7(AsO3)12·4H2O
[J]. Journal of Raman Spectroscopy, 2010, 41(3): 325-328. DOI: 10.1002/jrs.2325.Experimental and theoretical vibrational spectroscopic evaluation of arsenate coordination in aqueous solutions, solids, and at mineral-water interfaces
[J]. Geochimica et Cosmochimica Acta, 1998, 62(19-20): 3285-3300. DOI: 10.1016/S0016-7037(98)00222-1.Chemical oxidation of arsenopyrite using a novel oxidant: Chlorine dioxide
[J]. Minerals Engineering, 2019, 139: 105863. DOI: 10.1016/j.mineng.2019.105863.The oxidative dissolution of arsenopyrite (FeAsS) and enargite (Cu3AsS4) by Leptospirillum ferrooxidans
[J]. Geochimica et Cosmochimica Acta, 2008, 72(23): 5616-5633. DOI: 10. 1016/j.gca.2008.09.008.Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials
[J]. Applied Surface Science, 2008, 254(8): 2441-2449. DOI: 10.1016/j.apsusc.2007. 09.063.Arsenopyrite bio-oxidization behavior in bioleaching process: Evidence from laser microscopy, SEM-EDS, and XPS
[J]. Frontiers in Microbiology, 2020, 11: 1773. DOI: 10.3389/fmicb.2020.01773.Characterization of a natural and an electro-oxidized arsenopyrite: A study on electrochemical and X-ray photoelectron spectroscopy
[J]. International Journal of Mineral Processing, 2002, 65(2): 83-108. DOI: 10.1016/S0301-7516(01)00059-X.Leaching mechanism and electrochemical oxidation on the surface of chalcopyrite in ammonia–ammonium chloride solution
[J]. Journal of the Electrochemical Society, 2018, 165(10): E466-E476. DOI: 10.1149/2.0711810jes.Interaction mechanism of ferrate(VI) with arsenopyrite surface and its effect on flotation separation of chalcopyrite from arsenopyrite
[J]. Transactions of Nonferrous Metals Society of China, 2022, 32(11): 3731-3743. DOI: 10.1016/S1003-6326(22)66053-9.Arsenopyrite oxidation-A review
[J]. Applied Geochemistry, 2009, 24(12): 2342-2361. DOI: 10.1016/j.apgeochem.2009.09.008.Characterisation of sphalerite and pyrite surfaces activated by copper sulphate
[J]. Minerals Engineering, 2017, 100: 223-232. DOI: 10.1016/j.mineng. 2016.11.005.XPS analysis of bio-organic systems
[J]. Surface and Interface Analysis, 2011, 43(12): 1453-1470. DOI: 10.1002/sia.3831.XPS analysis of food products: Toward chemical functions and molecular compounds
[J]. Surface and Interface Analysis, 2008, 40(3-4): 718-724. DOI: 10.1002/sia.2779.Renewable nitrogen-containing products by Maillard reaction of sewage sludge and glucose. Part I. Analysis of nitrogen composition and protein model based on AlphaFold2
[J]. Fuel, 2022, 325: 124968. DOI: 10.1016/j.fuel.2022.124968.Nitrogen containing functional groups of biochar: An overview
[J]. Bioresource Technology, 2020, 298: 122286. DOI: 10.1016/j.biortech.2019.122286.Amino modification of biochar for enhanced adsorption of copper ions from synthetic wastewater
[J]. Water Research, 2014, 48: 396-405. DOI: 10.1016/j.watres.2013.09.050.Comparison of selected characteristics of Sulfobacillus species and review of their occurrence in acidic and bioleaching environments
[J]. Hydrometallurgy, 2008, 93(1, 2): 57-65. DOI: 10.1016/j.hydromet.2008.03.001.Evaluation of Leptospirillum spp. in the Río Tinto, a model of interest to biohydrometallurgy
[J]. Hydrometallurgy, 2008, 94(1-4): 155-161. DOI: 10.1016/j.hydromet.2008.05.046.Study on pre-oxidation of a high-arsenic and high-sulfur refractory gold concentrate with potassium permanganate and hydrogen peroxide
[J]. Transactions of the Indian Institute of Metals, 2020, 73(3): 577-586. DOI: 10.1007/s12666-020-01863-6.Gold extraction using alternatives to cyanide: Ultrasonic reinforcement and its leaching kinetics
[J]. Minerals Engineering, 2023, 191: 107939. DOI: 10.1016/j.mineng.2022.107939.Bioleaching and dissolution kinetics of pyrite, chalcocite and covellite
[J]. Journal of Central South University, 2021, 28(7): 2037-2051. DOI: 10.1007/s11771-021-4751-5.Sulfur chemistry, biofilm, and the (in)direct attack mechanism: A critical evaluation of bacterial leaching
[J]. Applied Microbiology and Biotechnology, 1995, 43(6): 961-966. DOI: 10.1007/BF00166909.(Bio)chemistry of bacterial leaching: Direct vs. indirect bioleaching
[J]. Hydrometallurgy, 2001, 59(2, 3): 159-175. DOI: 10.1016/S0304-386X(00)00180-8.Kinetics of sphalerite bioleaching by Acidithiobacillus ferrooxidans
[J]. Hydrometallurgy, 2009, 99: 202-208. DOI: 10.1016/J.HYDROMET.2009.08.007.ZHANG Shi-qi, YANG Hong-ying, TONG Lin-lin, CHEN Guo-min, KANG Guo-ai, and ZHAO Zhi-xin declare that they have no conflict of interest.
ZHANG Shi-qi, YANG Hong-ying, TONG Lin-lin, CHEN Guo-min, KANG Guo-ai, ZHAO Zhi-xin. Enhanced oxidation mechanism of arsenopyrite in two-stage oxidation process applying bio-oxidation waste solution [J]. Journal of Central South University, 2025, 32(1): 94-105. DOI: https://doi.org/10.1007/s11771-025-5862-1.
张仕奇,杨洪英,佟琳琳等.应用生物氧化废液的两段氧化工艺中毒砂的强化氧化机制[J].中南大学学报(英文版),2025,32(1):94-105.

