1 Introduction
Energy storage plays a critical role in ensuring the continuous availability of renewable energy sources [1-3]. The most common energy storage devices are lithium-ion batteries and supercapacitors. Lithium-ion batteries, despite their high energy density, have a disadvantage due to their limited lifespan and losses during charge-discharge cycles [4, 5]. On the other hand, supercapacitors offer an advantage with their longer lifespan and fast charge-discharge capability [6].
Supercapacitors store energy by either adsorbing ions in the electrolyte onto the electrode surface or undergoing redox reactions, making it desirable for electrode materials to have both a high surface area and redox-active components [7-9]. Therefore, compounds of metals like copper and iron are highly preferred due to their oxidizing/reducing properties. These metals are commonly sourced from ores such as pyrite and chalcopyrite [10, 11]. These copper and iron metals are extracted from these ores by extracting the acidic, basic, and salt components. However, the sulfur (S0) layer on the surface of chalcopyrite creates passivation, hindering the extraction of metals [12, 13]. In this context, TURAN and their colleagues attempted to overcome this by adding NaCl to the ammonium persulfate solution. They observed that the chloride ions in this solution disrupted sulfur passivation, resulting in copper and iron extraction efficiencies of 75% and 80% after 180 min duration, respectively [14]. VELÁSQUEZ-YÉVENES et al [15] treated chalcopyrite ore with high concentrations of acid and chloride ions, observing a 60% increase in copper extraction after a 20-day waiting period, with this increase continuing gradually with the waiting time. PETROVIC and their colleagues used hydrochloric acid and hydrogen peroxide to remove passivation. After an 80-min reaction period, they found that the maximum copper extraction rate was 33% with 0.5 mol/L HCl and 3.0 mol/L H2O2 at room temperature [16]. SolÍS-MARCIAL et al [17] argued that adding organic solvents to the solution is an effective method for removing the passivation layer. To achieve this, they employed various oxidants (H2O2, CuSO4, and O3) in an acidic solution and carried out chalcopyrite leaching at 40 ℃ in the presence of alcohols such as 2-propanol and hydrogen peroxide, determining an activation energy of 42 kJ/mol for copper dissolution. They also noted that above 40 ℃, peroxide degradation occurred, leading to a reduction in copper extraction. Additionally, they highlighted that methanol stabilized copper ions and prevented the formation of Cu2S2-6Cu2S. In all of these studies, the disruption of the passivation layer with secondary ions like oxidants has been successful in drawing copper and iron ions into the solution with primary components. Primary components such as salts like NaCl or MgCl2 are easily accessible, non-toxic, and cost-effective [18]. However, comparative analyses of different types of these salts are quite rare in the literature. On the other hand, sulfur is known to be removable with salts. Therefore, sulfur moving away from the surface can be captured by reacting with urea (CH4N2O) in the environment, converting it into thiourea (CH4N2S). This process may facilitate the transition of copper and iron into the solution.
On the other hand, such leach solutions are rich in copper and iron, and in the industry, it is crucial to separate both elements, which requires a challenging process. Instead, it is considered that the obtained leach solution, containing both copper and iron, can be used in a production where their combined is required, thus eliminating the need for separation [19, 20]. In this context, this leach solution can be utilized in the synthesis of spinel-structured CuFe2O4 [21], which has a widespread application as an electrode in supercapacitors, known for storing electrical energy. CuFe2O4 has high theoretical capacity of 895 mA·h/g [22], a low bandgap (1.9 eV) [23], and non-toxic properties, offering potential using as an electroactive material in supercapacitors [22, 24]. At the same time, these kinds of spinel-based ferrites provide reliable performance in industrial applications due to their high temperature resistance and stability in electromagnetic properties [25, 26]. Moreover, sulfur-containing components like thiourea are recognized to contribute to the increase in specific capacitance of electrodes. Therefore, the selection of a urea-containing component for CuFe2O4 synthesis may be an important preference due to these factors. Such components are typically attached to the current collector surface with a binder, which reduces the interaction at the electrolyte/electrode interface. However, it is possible to synthesize them in nanoscale on the current collector surface through a hydrothermal method from the leach solution [9]. In this regard, there is only one previous study in the literature, where different acids were used as extraction components [8].
The primary objective was to determine the optimal conditions for extracting the highest copper and iron content from chalcopyrite by varying the concentrations of NaCl, MgCl2, and urea, while taking into account the hypotheses outlined in this research. Subsequently, a CuFe2O4 material at nanoscale was synthesized using the urea contained leach solution, and it was deposited onto a nickel foam current collector surface using the hydrothermal method. The resulting products underwent comprehensive characterization through XRD, FT-IR, and SEM analyses. Their electrochemical performance was assessed through cyclic voltammetry (CV), galvanostatic charge-discharge (GCD), and impedance (EIS) measurements. Ultimately, this study’s uniqueness and contribution to the existing literature were demonstrated through a comparative analysis of the obtained results.
2 Experimental
2.1 Materials
The chalcopyrite particles employed in this study was 6 µm in mean size and came from the Kastamonu Hanönü region. Its chemical composition made by X-ray fluorescence (XRF) are given in Table 1. In the experiments, deionized water, sodium chloride (NaCl, 99.5% pure, EMPROVE®), magnesium chloride hekza hydrate (MgCl2.6H2O, 99% pure, EMSURE®) and urea (CH4N2O, %98 pure, Rokkim) were used for copper and iron extraction from chalcopyrite (CuFeS2). Nickel foam used for electrode substrate was purchased from Nanografi. Deionized water with a conductivity of 18.25 Ω was used for extraction.
| Fe | S | Cu | Zn | Si | Al | Co | Ca |
|---|---|---|---|---|---|---|---|
| 38 | 35 | 22 | 2.8 | 0.9 | 0.3 | 0.25 | 0.17 |
2.2 Copper and iron extraction from chalcopyrite
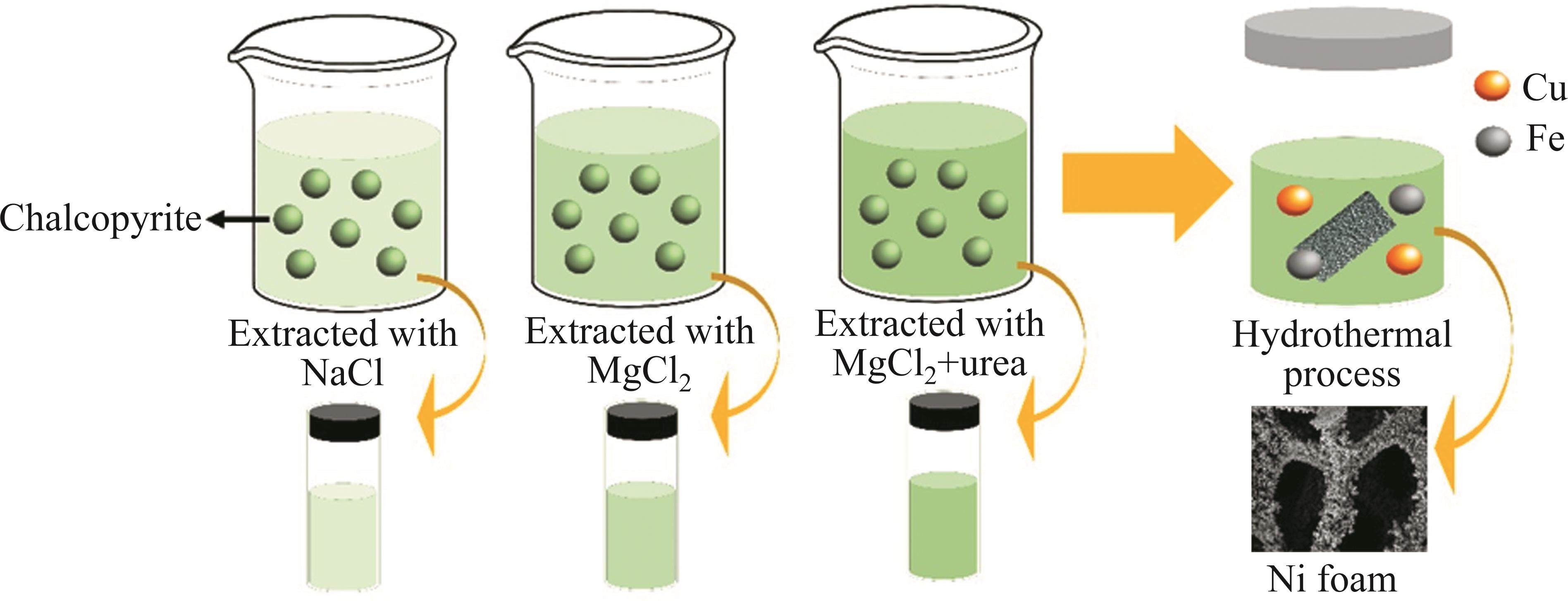
The leaching of chalcopyrite for obtaining Cu and Fe was applied by hydrometallurgical process [27]. A 250 mL beaker was initially used, and 50 mL of deionized water was added to it. The mixture in the beaker was then heated and stirred magnetically until it reached a temperature of 60 ℃. Once the temperature reached this point, 3 g of chalcopyrite were carefully weighed and added to the beaker. Stirring was maintained at the same temperature and a rate of 360 r/min for one hour. Following this step, the mixture was removed from the heater and filtered through Watman filter paper using a funnel. The filtrate was subsequently centrifuged at 3000 r/min for 15 min to eliminate any remaining particles, completing the extraction process. Additionally, NaCl and MgCl2 were individually used as secondary reactants to assess their impact on the concentrations of Cu and Fe elements in the extraction solution. These reactants were introduced into the solution alongside the chalcopyrite, following the quantities listed in Table 2. The quantities of NaCl and MgCl2 that yielded. The highest Cu and Fe concentrations were then determined. Afterward, urea was added in accordance with the amounts specified in this table, along with the highest concentration of MgCl2. All of these procedures were carried out using the setups illustrated in Figure 1.
| m(NaCl)/g | m(MgCl2)/g | m(MgCl2+Urea)/g | Duration/min | Stirring speed/(r·min-1) | Temperature/℃ |
|---|---|---|---|---|---|
| 0 g | 0 | 7+0 (M7) | 60 | 360 | 60 |
| 1 (N1) | 1 (M1) | 7+1 (M7U1) | 60 | 360 | 60 |
| 3 (N3) | 3 (M3) | 7+3 (M7U3) | 60 | 360 | 60 |
| 5 (N5) | 5 (M5) | 7+5 (M7U5) | 60 | 360 | 60 |
| 7 (N7) | 7 (M7) | 7+7 (M7U7) | 60 | 360 | 60 |
| 9 (N9) | 9 (M9) | 7+9 (M7U9) | 60 | 360 | 60 |
2.3 Preparation of electrodes
This section delineates the construction of electrodes for supercapacitors utilizing the solution with the highest concentrations of Cu and Fe, as specified in the characterization section. The fabrication procedure occurred on the surface of a nickel foam substrate as conducted our previous studies [8, 28], functioning as a direct current collector, employing the hydrothermal method. Initially, 10 mL of M7U1 solution was placed in a 40 mL Teflon-coated stainless-steel autoclave. Two pieces of nickel foam, cut to dimensions of approximately 1 cm2, were then inserted into the same autoclave. The Teflon lid and stainless-steel autoclave lid were tightly sealed. The autoclave was positioned in a preheated oven at 150 ℃ with a heating rate set to 10 ℃/min, allowing it to sit for 6 h. Afterward, the autoclaves were taken out of the oven and left to cool to room temperature. The nickel foams were detached from the lids, rinsed with deionized water to eliminate any remaining residues, and subsequently dried in an oven at 60 ℃ for one day. Furthermore, the autoclave solutions were utilized in characterization processes. These solutions were transferred to glass tubes, then centrifuged at 3000 r/min for 15 min to separate particulate matter. The remaining liquid was discarded, and the precipitate-like particles were dried in an oven for one day. Thus, the electrode fabrication processes were successfully concluded. This detailed representation of the narratives is schematically presented in Figure 1.
2.4 Materials characterization
In the initial phase of characterizations, the copper and iron concentrations in the post-extraction solutions were quantified using THERMO SCIENTIFIC ICE 3400 atomic absorption spectrometers (AAS). Subsequently, the characterizations delved into the crystallographic analysis of metal oxides synthesized through the hydrothermal method. These investigations were conducted using Rigaku Ultima IV X-ray diffraction (XRD) equipment, employing fixed monochromator Cu-based Kα radiation at 40 kV and 40 mA. Furthermore, Fourier transform infrared spectroscopy (FTIR) analyses were performed on these metal oxide compounds, covering a wave number range of 400 to 4000 cm-1, with a resolution of 2 cm-1, to elucidate their chemical bonds. Additionally, the microstructure and morphological images of these components were determined using the Carl Zeiss ULTRA PLUS scanning electron microscope (SEM).
2.5 Materials characterization
The electrochemical performance of hydrothermally synthesized electrodes was assessed using an Ametek Parstat 4000 device through cyclic voltammetry (CV) and galvanostatic charge-discharge (GCD). All electrochemical experiments were conducted using a three-electrode setup. In this configuration, a sample prepared via the hydrothermal process was employed as the working electrode, while a graphite rod and an Ag/AgCl electrode were used as the counter and reference electrodes, respectively. The electrolyte solution consisted of a 6 mol/L KOH solution prepared with deionized water (18.25 Ω). Portions of these nickel foams, measuring 1 cm², were immersed in the electrolyte after being compressed at approximately 10 MPa. The specific capacitance (Cs), energy (E) and power (P) densities of the electrodes were determined by Eqs. (1), (2), (3) [29]. In these equations, I, Δt, ΔV and S were current (mA), discharge duration (s), window (V) and surface area (cm2) of electrodes, respectively.
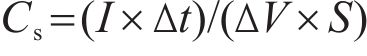
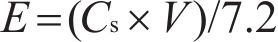
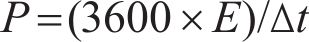
3 Result and discussion
3.1 Extraction results
The copper and iron extraction results obtained from different leach solutions of chalcopyrite have been examined in this section. The concentrations of the leach solutions using the NaCl reactant are detailed in Figures 2(a) and (b). The samples referred to as “reference” in these results were generated using only deionized water, without the addition of any reactants. Based on these results, the Cu and Fe concentrations in the reference sample were determined to be 225 and 203 mg/L, respectively. In the following experiments, 1 g of NaCl was added as a reactant (N1) to the extraction solution, which led to Cu and Fe concentrations of 295 and 199 mg/L, respectively. Subsequently, another leach solutions containing 3 g (N3), 5 g (N5), 7 g (N7) and 9 g (N9) of NaCl were prepared to assess changes in the extraction rate with varying reactant quantities. Cu concentrations in these solutions were measured as 306, 333, 380 and 400 mg/L, respectively, while Fe concentrations were found to be 205, 221, 242 and 261 mg/L. These findings indicate that Cu concentrations increased by 28% to 76%, and Fe concentrations similarly increased by 28% to 76%, depending on the amount of NaCl added. This increase is likely related to the deformation of the sulphur layers in chalcopyrite by Na+ ions. However, the point of saturation in terms of Cu and Fe concentrations, depending on the amount of NaCl, could not be determined. Instead, the increasing trends of Cu and Fe concentrations were identified, with slopes of 0.86 and 0.34, respectively. These results have also been compared with the literature in the discussion section.
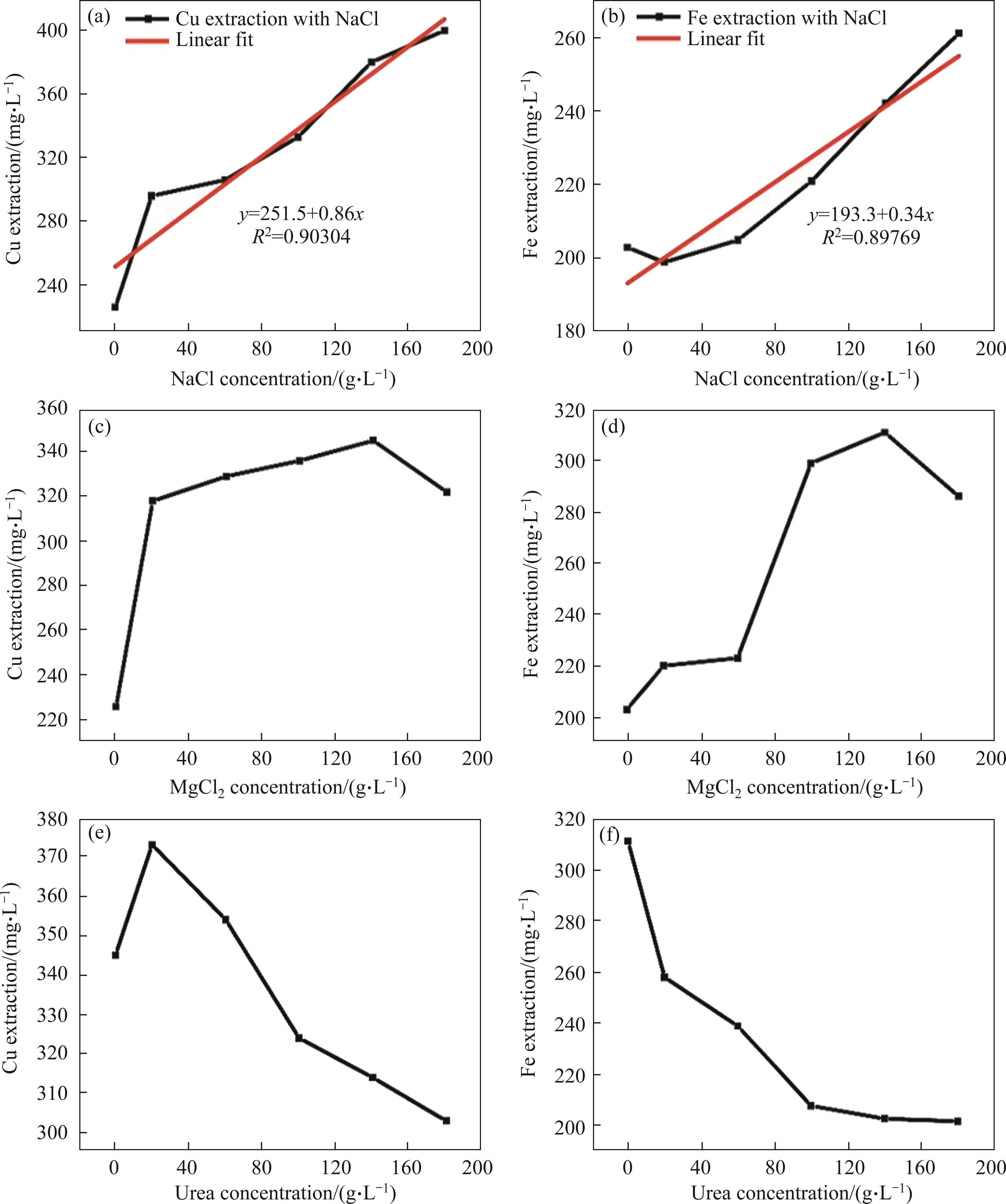
The effect of MgCl2 as a second reactant has been investigated. For this purpose, leach solutions containing 1, 3, 5, 7 and 9 g of MgCl2 were similarly prepared and labelled as M1, M3, M5, M7 and M9, respectively. The Cu and Fe concentrations of each solution were provided in Figures 2(c) and (d). According to findings, the Cu concentrations for M1, M3, M5, M7 and M9 were determined to be 318, 329, 336, 345 and 322 mg/L, respectively, while the Fe concentrations was measured at 220, 223, 299, 311 and 286 mg/L. The concentrations of Cu and Fe showed an increase of approximately 52%-53% until the M7 leach solution, after which they exhibited a decreasing trend. This M7 leaching solution can be considered as the point where Cu and Fe reach saturation. On the other hand, the effect of another reactant, urea (CH4N2O), an organic compound along with these salts, was also examined but, since no effect of urea was observed in combination with NaCl, it was not considered for evaluation. However, when used with MgCl2, the changes observed were presented in Figures 2(e) and (f). The urea component was added to the M7 leach solution, which yielded the highest Cu and Fe concentrations, in quantities of 1, 3, 5, 7 and 9 g, respectively. These leach solutions prepared with these components were labelled as M7U1, M7U3, M7U5, M7U7 and M7U9, as shown in Table 2. The Cu concentrations for the M7U1, M7U3, M7U5, M7U7, and M7U9 samples were determined as 345, 373, 354, 324, 314 and 303 mg/L, respectively. Similarly, the Fe concentrations were found to be 311, 258, 239, 208, 203 and 202 mg/L. When compared to the M7 sample, these results showed an initial 8% increase for Cu, followed by a subsequent decrease of up to 12% in the M7U9 sample. Concurrently, Fe concentrations exhibited a direct decrease with the introduction of urea, reaching up to 35%. Therefore, the urea compound might be considered a suitable candidate for obtaining copper without iron, especially when MgCl2 is used instead of NaCl.
3.2 Electrode characterization
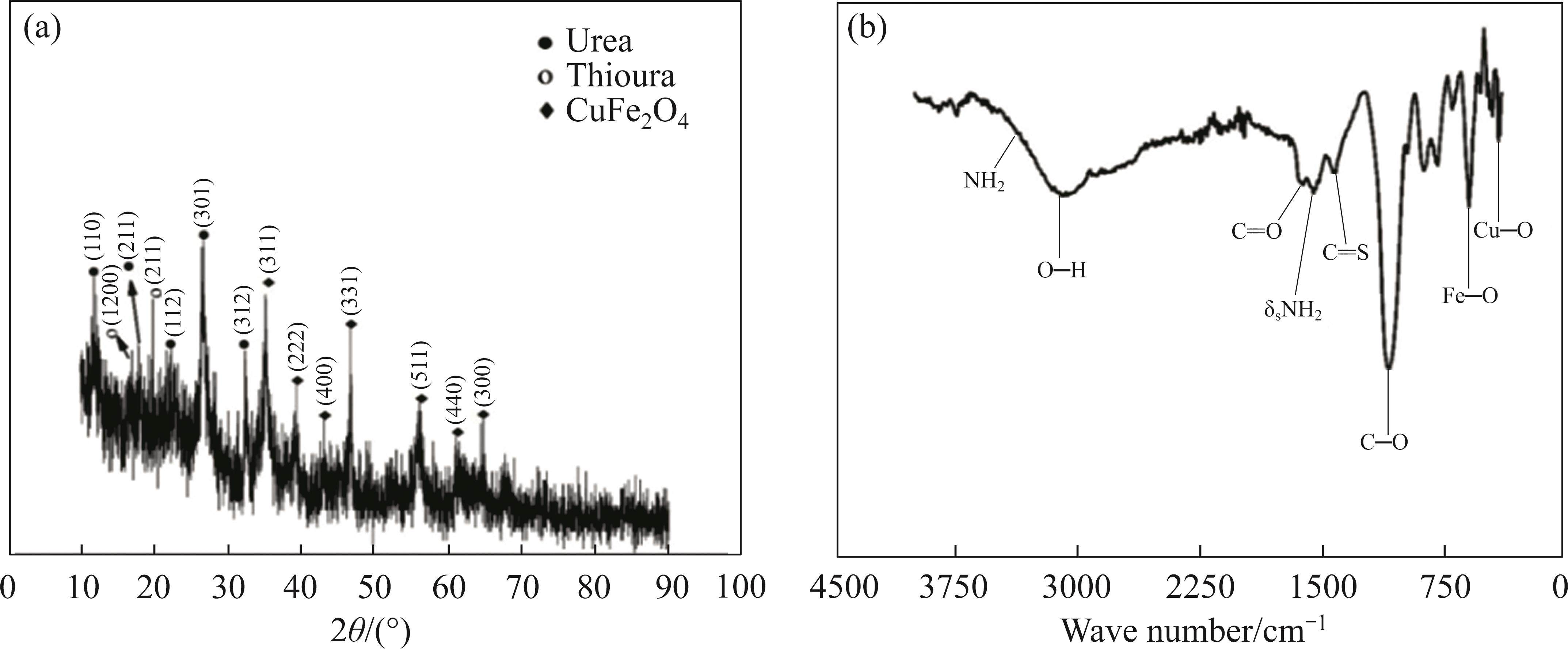
The synthesis of CuFe2O4 metal oxide for supercapacitors was planned using copper and iron ions present in the leach solution obtained after extraction. For this purpose, the M7U1 solution was selected, considering that the thiourea formed after leaching might advantageously contribute to the electrode’s performance. The productions were carried out directly on a nickel foam substrate via hydrothermal method, and the obtained products were initially subjected to XRD analyses, as shown in Figure 3(a). According to the results, distinct crystal peaks were observed at 2θ values of 35°, 39°, 43°, 55°, 60° and 64°, which are likely attributed to the (311), (222), (400), (511), (440) and (300) planes of the spinel-structured CuFe2O4 [30, 31]. The majority of these peaks are also consistent with JCPDS data (Card No. 22—1086) of CuFe2O4. Simultaneously, additional peaks were detected at 11°, 17°, 22°, 26° and 32°, which are likely attributed to the (110), (211), (112), (301) and (312) planes of urea, as well as peaks at 16° and 17°, 19°, corresponding to the (200) and (211) planes of thiourea, respectively [32]. Furthermore, the FT-IR results, presented in Figure 3(b), revealed two distinct peaks at wave numbers 456 and 598 cm-1, indicative of Cu—O and Fe—O bonds, respectively [7, 33]. Additionally, supplementary peaks were observed at 1082, 1409, 1558, 1627 and 598 cm-1, suggesting the presence of carbon-like and NH2 compounds. Likewise, the broad peak observed at 3095 cm-1 is associated with O—H bonding, likely attributed to moisture within the structure [14].
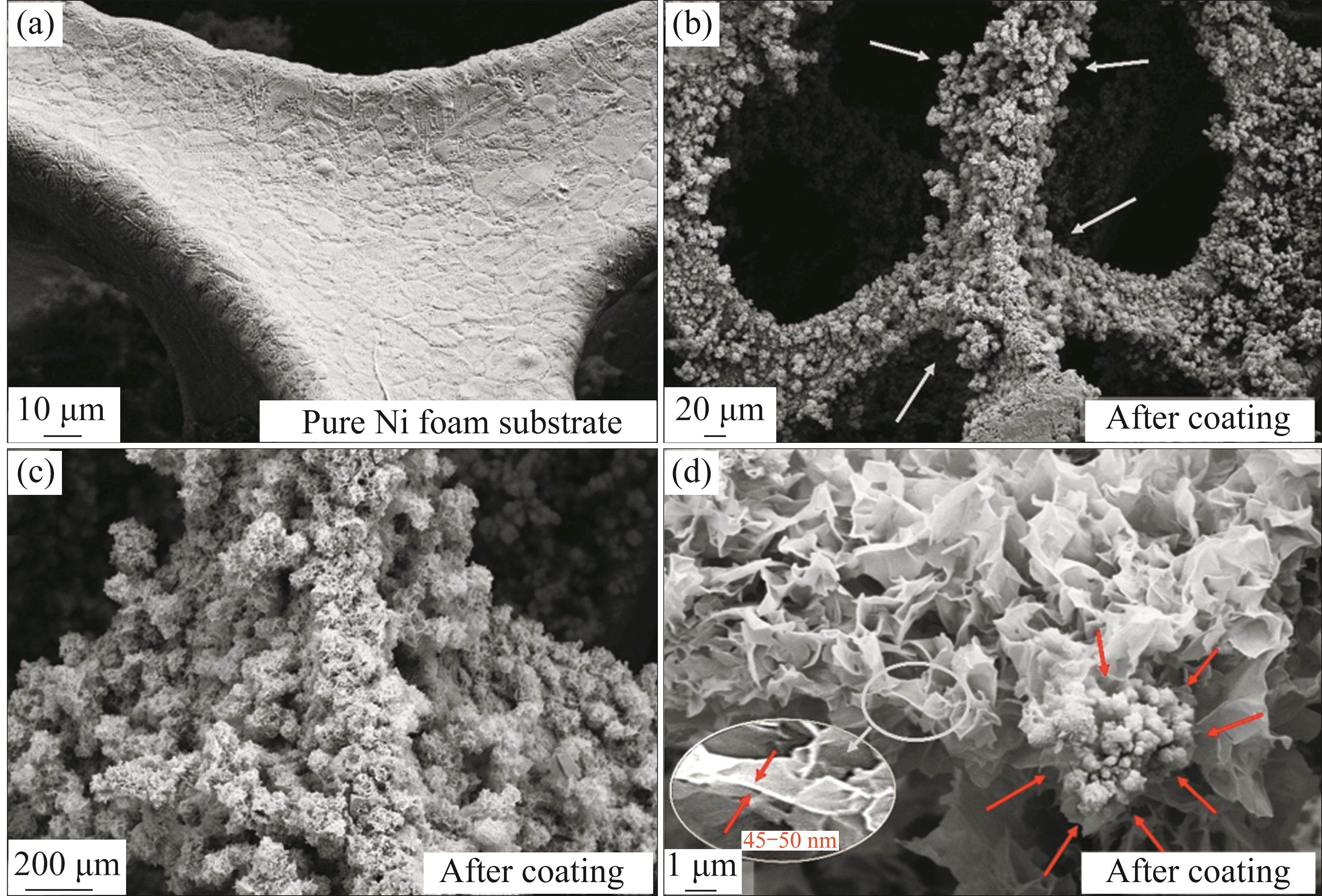
The microstructure of metal oxide nanoparticles based on Cu and Fe, synthesized on the surface of nickel foam, was examined using SEM, and the results are presented in the visuals within Figure 4. An overview of the untreated and treated nickel foam is provided in Figures 4(a) and (b), respectively. These images clearly show the formation that has taken place on the surface of the nickel foam. In the higher magnification images shown in Figures 4(c) and (d), it is evident that these formations exhibit a plate-like structure depicted in Figure 4(c). It can be reasonably approximately 45-50 nm wall thickness as inferred that these structures, characterized by their remarkably thin walls and nano-scale dimensions, primarily consist of CuFe2O4 crystal structures, as corroborated by the XRD and FT-IR analyses. This interpretation is further supported by relevant literature data.
3.3 Electrode characterization
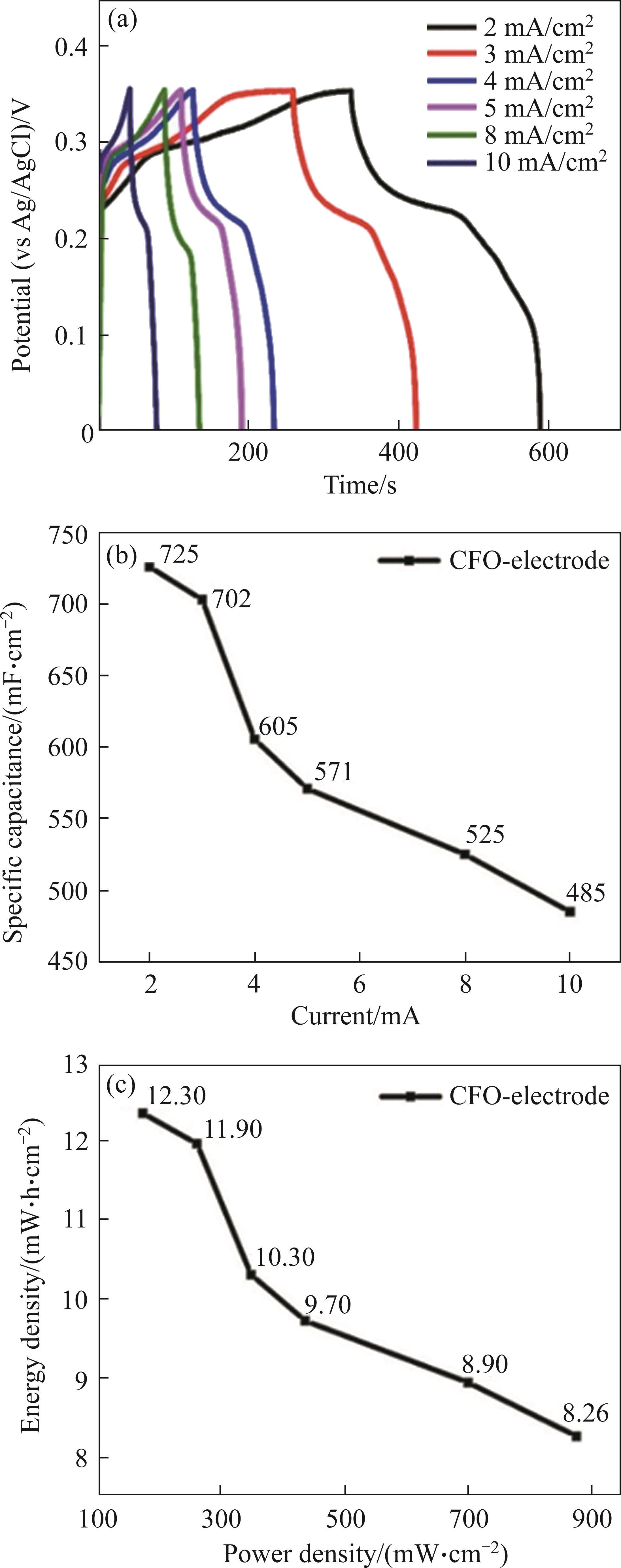
The synthesis of Cu-Fe metal oxide nanoparticles directly on the nickel foam surface via the hydrothermal method [2, 34], using the extraction solution, was confirmed through XRD, FT-IR, and SEM analyses. The resulting samples were intended for use as anode electrodes in energy storage applications, particularly in supercapacitors. In this context, we conducted investigations into the electrochemical performance of nickel foam electrodes coated with metal oxide nanoparticles on their surface. For this purpose, galvanostatic charge-discharge measurements were performed at various current densities, specifically 2, 3, 4, 5, 8 and 10 mA/cm2, and the outcomes are presented in Figure 5(a). The corresponding discharge durations at these current densities were recorded as 251 s, 163 s, 107 s, 82 s, 46 s and 35 s, respectively. Furthermore, based on these discharge time, the specific areal capacitance values (Ca) were computed at each current level, resulting in values of 725, 702, 605, 571, 525 and 485 mF/cm2, as illustrated in Figure 5(b). Notably, the highest energy storage performance was achieved at a current density of 2 mA/cm2. Additionally, we calculated the energy and power densities of the electrode, and these results are depicted in Figure 5(c). According to our findings, the energy densities for each current density, ranging from 2 to 10 mA/cm2, were determined to be 12.30, 11.90, 10.30, 9.70, 8.90 and 8.26 mW·h/cm², respectively. Simultaneously, the power density of the electrode, within the same current range, was computed as 175, 262, 350, 437, 700 and 875 mW/cm², as demonstrated in Figure 5(c). Consistent with the trend observed in specific capacitance, the highest energy and power density values were obtained at a current density of 2 mA/cm2.
3.4 Discussion
Copper and iron are two elements widely used in industry. Mineralogists conduct a series of studies to separately extract these elements from the chalcopyrite ore, where they are found together. These studies generally focus on various parameters such as the type of reactants, ambient temperature, duration, solid-liquid ratio, and pH. A brief summary of the studies conducted on chalcopyrite ore can be found in Table 3 in the literature. In order to compare the findings of our study with those in the literature, extraction rates were calculated according to the formula in the reference study [21]. According to findings from literature, it was observed that increasing the solution temperature from 70 to 90 ℃ resulted in a 50% increase in copper extraction in one study focusing on temperature [10]. Regarding duration, it was stated that a leaching process of 180 min is sufficient to reach copper saturation [38]. However, the simultaneous increase in iron extraction along with copper during this period is a significant disadvantage. When examined in terms of reactant type, it was observed that the highest copper extraction occurred with NaCl (150 g/L) and APS (250 g/L), reaching 80% [38]. At the same time, copper extraction with H2SO4 (1 mol/L) and NaCl (2 mol/L) reactants was 70% [10], while with NaCl (1 mol/L) and H2SO4 (100 mL) it was 60% [37]. However, the absence of comparison with pure water and the lack of iron extractions in these studies are considered significant shortcomings. In our study, this deficiency was addressed by initially conducting the leaching process with pure water, where copper and iron extractions were determined to be 33% and 18% respectively. When 3 mol/L NaCl was added to the leach solution, copper and iron extractions reached 60% and 23% respectively, while with MgCl2 they were 52% and 27%. In terms of quantity, it was determined that copper and iron extractions continued to increase with NaCl addition, with slopes of increase being 0.86 and 0.34, respectively. However, for MgCl2, it was understood that copper and iron extractions decreased after a concentration of 140 g/L. Likely, this is because Na+ ions exhibit higher reactivity in forming sulphated compounds with sulphur compared to Mg+2 ions [39]. Additionally, it was observed that MgCl2 reduced copper extraction while increasing iron extraction, indicating a disadvantage for obtaining copper-free iron. Apart from these, it was considered that a secondary component such as urea reacting with the sulphur around the chalcopyrite could allow for greater solubility of copper and iron. However, no change was observed when added with NaCl, and thus it was not included in the study. When added with MgCl2, it was observed that only at a concentration of 20 g/L did it increase copper extraction while consistently reducing iron. The peak in copper extraction was determined to be 56%, which, although lower than NaCl, was relatively higher compared to pure MgCl2. Additionally, when urea was added with MgCl2, it likely formed a complex compound [40], reducing iron extraction, which could be suitable for obtaining copper-free iron. However, it is evident that this hypothesis requires further investigation.
| Reactant | Duration | Temperature/℃ | Others | Cu extraction | Fe extraction | Ref. |
|---|---|---|---|---|---|---|
| NaCl (0.5 mol/L) | 15 days | 30 | 10g/L CHA, 45 μm | ~256 mg/L | ~30 mg/L | [35] |
| MgCl2 (0.5 mol/L) | 15 days | 30 | 10g/L CHA, 45 μm | ~227 mg/L | — | |
NaCl (300 g/L) HCl (0.5 mol/L) CuCl2 (1 mol/L) | 100 min | 100 | 160 g/L CHA, 40 μm | — | ~29% | [36] |
NaCl-(1 mol/L) 100 mL H2SO4 | ~450 h | 55 | 1 g CHA, 38 μm | ~60% | — | [37] |
NaCl (150 g/L) APS (250 g/L) | 180 min | 60 | 250 mL/g CHA, ~75 μm | ~80% | ~75% | [38] |
H2SO4 (1 mol/L) NaCl (2 mol/L) | 180 min | 90 | 0.1 (w/v) CHA, ~19 μm | ~70% | — | [10] |
| Deionized water | 60 min | 60 | 300 g/L CHA, 6 μm | ~33% | ~18% | This study |
| NaCl (~3 mol/L) | 60 min | 60 | 300 g/L CHA, 6 μm | ~60% | ~23% | |
| MgCl2 (~0.68 mol/L) | 60 min | 60 | 300 g/L CHA, 6 μm | ~52% | ~27% | |
MgCl2+Urea (~0.68 mol/L) (~1.6 mol/L) | 60 min | 60 | 300 g/L CHA, 6 μm | ~56% | ~20% |
In the second part of the study, the counterparts of CuFe2O4-based electrodes produced for supercapacitors were investigated in the literature, and the findings were summarized in Table 4. According to these results, the specific capacitance value of the electrode in this study was observed to be approximately 725 mF/cm2 or (~504 F/g) at a current density of 2 mA/cm2. When comparing among CuFe2O4-based electrodes developed so far, it is evident that those with a 2D geometric shape outperform spherical ones. Furthermore, the addition of graphene-like components with high conductivity to CuFe2O4 leads to a noticeable improvement in performance [22]. A similar study conducted by incorporating graphene and graphitic carbon nitride in a 2D structure achieved high performance, approximately 989 mF/cm2 (~657 F/g) [7]. Now in this study, despite obtaining CuFe2O4 with only thiourea residues on its surface, remarkably high performance was still achieved. In fact, the results of this study were observed to surpass those obtained from a similar study using acidic leaching reagent solutions [8]. Therefore, the procedure in this study is considered promising and open to further development.
| Electrode materials | Electrolyte solution | Specific capacitance | Capacitance retention | Ref. |
|---|---|---|---|---|
CFO graphene g-C3N4 | 6 mol/L KOH | 989 mF/cm2 at 1.3 mA/cm2 (~657 F/g) | 73.3% after 1500 cycles | [7] |
| CFO | 6 mol/L KOH | 725 mF/cm2 at 2 mA/cm2 (~504 F/g) | — | This study |
CuFe2O4 graphene | 3 mol/L KOH | 576.6 F/g at 1 A/g | 85% after 1000 cycles | [22] |
| CuFe2O4 | 0.5 mol/L H2SO4 | 437.3 F/g at 4 mV/s | 88.6% after 2000 cycles | [41] |
| CuFe2O4 | 1 mol/L KOH | 345 F/g at 0.6 A/g | 88% after 600 cycles | [42] |
| CuFe2O4 | 2 mol/L KOH | 189.2 F/g at 0.5 A/g | 84% after 1000 cycles | [43] |
4 Conclusions
This study was conducted in two stages. In the first stage, the effect of NaCl and MgCl2 salts in combination with urea on the extraction of Cu and Fe from chalcopyrite was investigated. In the second stage, CuFe2O4 electrode synthesis for supercapacitors was carried out using the extraction solution containing Cu and Fe. The obtained results were evaluated in two stages as given below. The highest performance was observed with 3 mol/L NaCl, yielding 60% Cu extraction and 23% Fe extraction. On the other hand, 1.5 mol/L MgCl2 resulted in 52% Cu and 27% Fe extraction, while a combination of 0.5 mol/L MgCl2 and 1.6 mol/L urea achieved 57% Cu and 20% Fe extraction. Notably, urea was found to be effective in reducing iron levels in the solution.
CuFe2O4-based electrodes for supercapacitors were successfully synthesized using a hydrothermal method with a MgCl2-urea solution. Characterization of these electrodes through XRD, FT-IR, and SEM revealed the formation of CuFe2O4 with a 2D structure and wall thickness of approximately 45-50 nm on the surface of the nickel foam. Electrochemical analysis showed promising results, with a specific capacitance of 725 mF/cm² at 2 mA/cm2 current density. Additionally, energy and power densities reached 12.3 mW·h/cm² and 175 mW/cm², respectively, under the same current density. These findings collectively contribute to our understanding of copper and iron extraction mechanisms from chalcopyrite and offer insights into the potential application of CuFe2O4-based electrodes in supercapacitors, showcasing their promising electrochemical performance.
A comprehensive review of stationary energy storage devices for large scale renewable energy sources grid integration
[J]. Renewable and Sustainable Energy Reviews, 2022, 159: 112213. DOI: 10.1016/j.rser.2022.112213.Production of ZnFe2O4 doped carbon cloth-based flexible composite electrodes for supercapacitors
[J]. Türk Doğa ve Fen Dergisi, 2021, 10: 199-205. DOI: 10.46810/tdfd.953992.Spring assisted triboelectric nanogenerator based on sepiolite doped polyacrylonitrile nanofibers
[J]. Sustainable Energy Technologies and Assessments, 2021, 47: 101492. DOI: 10. 1016/j.seta.2021.101492.Advances and prospects in improving the utilization efficiency of lithium for high energy density lithium batteries
[J]. Advanced Functional Materials, 2023, 33: 2302055. DOI: 10.1002/adfm.20230 2055.High-energy batteries: Beyond lithium-ion and their long road to commercialisation
[J]. Nano-Micro Letters, 2022, 14(1): 94. DOI: 10.1007/s40820-022-00844-2.A comprehensive review on transition metal nitrides electrode materials for supercapacitor: Syntheses, electronic structure engineering, present perspectives and future aspects
[J]. Journal of Energy Storage, 2023, 72: 108229. DOI: 10.1016/j.est.2023.108229.Fabrication of CuFe2O4@g-C3N4@GNPs nanocomposites as anode material for supercapacitor applications
[J]. Ceramics International, 2022, 48(17): 24609-24618. DOI: 10.1016/j.ceramint.2022.05.106.Sustainable cauliflower-patterned CuFe2O4 electrode production from chalcopyrite for supercapacitor applications
[J]. Nanomaterials, 2023, 13(6): 1105. DOI: 10.3390/nano1306 1105.Synthesis and electrochemical performance of MgFe2O4 with g-C3N4 on Ni-foam as composite anode material in supercapacitors
[J]. Journal of Materials Science: Materials in Electronics, 2022, 33(30): 23427-23436. DOI: 10.1007/s10854-022-09104-w.Kinetics of chalcopyrite leaching in either ferric sulphate or cupric sulphate media in the presence of NaCl
[J]. International Journal of Mineral Processing, 2016, 148: 147-154. DOI: 10.1016/j.minpro.2016.01.014.Liquid copper and iron production from chalcopyrite, in the absence of oxygen
[J]. Metals, 2022, 12(9): 1440. DOI: 10.3390/met12091440.Thermodynamic interaction and iron passivation mechanism of copper-cobalt sulfide concentrates during pressure leaching
[J]. Chemical Papers, 2023, 77(5): 2737-2747. DOI: 10.1007/s11696-023-02663-0.Differential surface modification mechanism of chalcopyrite and pyrite by Thiobacillus ferrooxidans and its response to bioflotation
[J]. Bioresource Technology, 2024, 399: 130619. DOI: 10.1016/j.biortech.2024.130619.Improving of copper extraction from chalcopyrite by using NaCl
[J]. Journal of Central South University, 2018, 25(1): 21-28. DOI: 10.1007/s11771-018-3713-z.Leaching of chalcopyrite ore agglomerated with high chloride concentration and high curing periods
[J]. Hydrometallurgy, 2018, 181: 215-220. DOI: 10.1016/j.hydromet.2018.10.004.Leaching of chalcopyrite with hydrogen peroxide in hydrochloric acid solution
[J]. Transactions of Nonferrous Metals Society of China, 2018, 28(7): 1444-1455. DOI: 10. 1016/S1003-6326 (18)64788-0.Chalcopyrite leaching in alcoholic acid media
[J]. Hydrometallurgy, 2014, 147: 54-58. DOI: 10.1016/j.hydromet.2014.04.011.Recyclable recovery of lead from zinc hydrometallurgy residue: By NH4Cl-MgCl2 leaching and novel cyclone-electrowinning
[J]. Industrial & Engineering Chemistry Research, 2024, 63(2): 942-954. DOI: 10.1021/acs.iecr.3c0 2872.A review on the research of hydrometallurgical leaching of low-grade complex chalcopyrite
[J]. Journal of Sustainable Metallurgy, 2022, 8(3): 964-977. DOI: 10.1007/s40831-022-00561-5.Chalcopyrite leaching in novel lixiviants
[J]. Hydrometallurgy, 2022, 207: 105775. DOI: 10.1016/j.hydromet.2021.105775.Extraction efficiency of metals from low-nickel matte via NH4Cl roasting-water leaching process and synthesis of (Ni, Cu, Co)Fe2O4 photocatalyst
[J]. Journal of Central South University, 2023, 30(6): 1803-1816. DOI: 10.1007/s11771-023-5342-4.One-step facile solvothermal synthesis of copper ferrite-graphene composite as a high-performance supercapacitor material
[J]. ACS Applied Materials and Interfaces, 2015, 7(4): 2404-2414. DOI: 10.1021/am507014w.Photocatalytic removal of ciprofloxacin in water by novel sandwich-like CuFe2O4 on rGO/halloysite material: Insights into kinetics and intermediate reactive radicals
[J]. Water, 2023, 15(8): 1569. DOI: 10.3390/w15081569.Enhanced electrochemical activity of PVA assisted CuFe2O4 nanoparticles as a potential electrode for the fabrication of high energy density hybrid supercapacitor
[J]. Inorganic Chemistry Communications, 2023, 157: 111349. DOI: 10.1016/j.inoche.2023.111349.Comparison of microwave absorption properties between BaTiO3/epoxy and NiFe2O4/epoxy composites
[J]. Polymer Composites, 2018, 39(S4): E2143-E2148. DOI: 10.1002/pc.24497Symbiotic strategy of Cu on CuFe2O4 realizing high-efficiency electromagnetic wave absorption
[J]. Journal of Colloid and Interface Science, 2023, 645: 841-849. DOI: 10.1016/j.jcis. 2023.04.141.Eco-friendly oxidation leaching from chalcopyrite powder and kinetics assisted by sodium chloride in organic acid media
[J]. Advanced Powder Technology, 2022, 33(5): 103547. DOI: 10.1016/j.apt.2022.103547.Synthesis of zinc oxide nanorods from zinc borate precursor and characterization of supercapacitor properties
[J]. Nanomaterials, 2023, 13(17): 2423. DOI: 10.3390/nano13172423.Hydrothermal synthesis and electrochemical performance of GNPs-doped MgFe2O4 electrodes for supercapacitors
[J]. Solid State Ionics, 2023, 391: 116107. DOI: 10.1016/j.ssi.2022.116107.ZnFe2O4 and CuFe2O4 nanocrystals: Synthesis, characterization, and bactericidal application
[J]. Journal of Cluster Science, 2023, 34(1): 111-119. DOI: 10. 1007/s10876-021-02203-4.Pure and Ce-doped spinel CuFe2O4 photocatalysts for efficient rhodamine B degradation
[J]. Environmental Research, 2021, 200: 111528. DOI: 10.1016/j.envres.2021.111528.XRD and FTIR studies of thiourea urea potassium iodide single crystal
[J]. Int J Res Eng Appl Manag, 2018, 4: 187-191. DOI: 10.18231/2454-9150.2018. 0608.Magnetic recyclable CuFe2O4/rGO nanocomposite for the degradation of tetracycline under sunlight irradiation
[J]. Nature, 2017, 68: 30-31. DOI: 10.1038/068030b0.Microwave hydrothermal synthesis, characterization and excellent uranium adsorption properties of CoFe2O4@rGO nanocomposite
[J]. Journal of Central South University, 2021, 28(7): 1955-1965. DOI: 10.1007/s11771-021-4744-4.Synergistic catalytic effect of chloride ion and ammonium ion on the leaching of chalcopyrite in sulfuric acid solution
[J]. Minerals Engineering, 2022, 185: 107686. DOI: 10.1016/j.mineng.2022.107686.Effect of NaCl concentration and particle size on chalcopyrite leaching in cupric chloride solution
[J]. Hydrometallurgy, 2005, 77(1, 2): 109-114. DOI: 10.1016/j.hydromet.2004.10.015.An improved understanding of chalcopyrite leaching kinetics and mechanisms in the presence of NaCl
[J]. Journal of Materials Research and Technology, 2019, 8(4): 3487-3494. DOI: 10.1016/j.jmrt. 2019.06.020.Improving of copper extraction from chalcopyrite by using NaCl
[J]. Journal of Central South University, 2018, 25(1): 21-28. DOI: 10.1007/s11771-018-3713-z.The effects of chloride on the high-temperature pressure oxidation of chalcopyrite: Some insights from batch tests: Part 1: Leach chemistry
[J]. Minerals, 2023, 13(8): 1065. DOI: 10.3390/min13081065.Improving flotation separation of Mg(OH)2-depressed chalcopyrite and pyrite by EDTA modification: A promising method for sustainable Cu production
[J]. J Sustain Metall, 2023, 9: 1647-1659. DOI: 10.1007/s40831-023-00754-6.Tween modified CuFe2O4 nanoparticles with enhanced supercapacitor performance
[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 631: 127676. DOI: 10.1016/j.colsurfa.2021.127676.Facile fabrication of hierarchically porous CuFe2O4 nanospheres with enhanced capacitance property
[J]. ACS Applied Materials and Interfaces, 2013, 5(13): 6030-6037. DOI:10.1021/am4007353.Electrochemical performances of monodispersed spherical CuFe2O4 nanoparticles for pseudocapacitive applications
[J]. Vacuum, 2019, 168: 108798. DOI: 10.1016/j.vacuum.2019.108798.POLAT Safa, MOHAMMED Mariem, MASHRAH Muwafaq declare that they have no conflict of interest.
POLAT Safa, MOHAMMED Mariem, MASHRAH Muwafaq. Copper and iron extraction from chalcopyrite by NaCl@MgCl2@urea: Synthesis of CuFe2O4 electrodes for supercapacitors [J]. Journal of Central South University, 2025, 32(1): 82-93. DOI: https://doi.org/10.1007/s11771-025-5860-3.
.利用NaCl@MgCl2@尿素萃取黄铜矿中的Cu、Fe并合成超级电容器用CuFe2O4电极[J].中南大学学报(英文版),2025,32(1):82-93.

