1 Introduction
Lead (Pb) and zinc (Zn) are prevalent main contaminants in soil, due to intensive cultivation and industrial activities, such as high input of Zn-containing chemical fertilizers and pesticides, wastewater irrigation, uses of Pb-containing paints and gasoline, chemical manufacturing, mining, and smelting [1-3]. Although Zn is plant essential micronutrient, acting as structural and enzymatic activation, oversupply of it could cause toxicity. Pb and Zn result in soil degradation by altering the normal ecosystem structure and functioning and inducing toxicity in vegetation and microbial community [1, 4, 5]. Pb and Zn could result in oxidative stress, toxicity and surplus accumulation of reactive oxygen species (ROS) in plants, which can damage DNA and RNA, inhibit synthesis and activities of enzyme and protein, and destroy antioxidant enzymes system in plant cells [6]. Green vegetables grown in Pb and Zn contaminated soils are eaten by humans through the food chain, resulting in threats to human health, such as lung cancer, abdominal pain, kidney failure and stomach trouble [7]. Thus, effective, economical and eco-friendly immobilization strategies are urgently required for Pb and Zn contamination.
Biochar has been considered as a promising inoculant carrier for beneficial microorganisms, due to its large surface aera, highly porous structure, and abundant nutrients and surface functional groups [8, 9]. Our previous work presented that inorganic phosphate-solubilizing bacteria (IPSB) immobilized on biochar could successfully transform labile Pb to stable state, reducing Pb toxicity in soil [10]. In addition, immobilized sulfate reducing bacteria (SRB) on biochar had the largest passivate performance, reducing the bioavailable Zn fraction by 22.2% in the Zn concentration of 1500 mg/kg in soil [11]. It was reported that IPSB encapsulated by sodium alginate containing Ca3(PO4)2 could reinforce Pb passivation effects in sediments by ZHANG et al [12], and the removal efficiencies for 100 mg/L initial concentration of Cu2+ and Zn2+ were 98.17% and 99.67%, respectively, in SRB loaded on Cu and Fe particles after 48 h by ZHOU et al [13]. However, how amendments of complex bacteria loaded biochar alleviated Pb and Zn stress on plants still remains not clear. Moreover, little is known about interactive effects of bacteria consortia and biochar on the physiological and biochemical responses of plant for complex Pb and Zn stresses.
Therefore, we hypothesized that 1) biochar, IPSB, acinetobacter calcoaceticus (A. calcoaceticus) and SRB (Enterobacter sp.) consortia, and the combination consortia immobilized biochar could work well in immobilizing complex Pb and Zn in soil and reducing the Brassica uptake for Pb and Zn; 2) three amendments may protect photosynthetic apparatuses from Pb and Zn toxicity; 3) three amendments may ameliorate the damage of Pb and Zn to antioxidant enzyme system. Thus, the aim of this study was to: 1) investigate the synchronous immobilization effects of biochar, bacteria consortia and bacteria consortia loaded biochar on Pb and Zn; 2) analyze the effects on growth, photosynthesis characteristics, antioxidant enzymes and Pb and Zn content of Brassica; 3) explore interactive effects on the physiological and biochemical responses of Brassica for Pb and Zn stresses. Our results may provide understanding for link soil health and plant growth to soil functioning resilience and establishment of sustainable agroecosystems.
2 Materials and methods
2.1 Soil characteristics
Each pot was filled with 10 kg of air-dried, sieved (2 mm), and homogenized contaminated soil by Pb and Zn. Soil was collected from the Northwest University experimental field located in Xi’an City, Shaanxi Province, China, then spiked with different concentrations of PbNO3 and ZnCl2 to obtain final Pb+Zn concentration of 300+250, 900+750 and 1800+1500 mg/kg, respectively [11]. The non-spiked soil was classified as clay loam and its basic physicochemical properties were pH of 8.12, available phosphorus (AP) of 2.66 mg/kg and cation exchange capacity (CEC) of 172.18 cmol/kg.
2.2 Soil amendments
In our previous work, biochar serving as target carrier was produced from wheat straw under limited oxygen condition and was denoted as WS700. WS700 has a specific surface area of 71.2 m2/g, pH of 10.07, and an ash rate of 26.53%. Inorganic phosphate-solubilizing bacteria (A. calcoaceticus) and sulfate reducing bacteria (Enterobacter sp.) were isolated from lead-zinc mine soils located in Feng County. A. calcoaceticus could dissolve phosphate of 531.28 mg/L, denoting as IPSB, and Enterobacter sp. was denoted as SRB with sulfate reduction efficiency of 92.8%. The detailed incubation experiments and the properties of WS700, IPSB and SRB could be found in our previous work [10, 11].
2.3 Experimental plant
The bok choy, Brassica rapa var. chinensis, was purchased from Taobao No. 1 Farm Seed Industry. Each pre-remediated pot was transplanted into five Brassica seedlings with similar heights and developed four leaves. Moisture content of each pot was kept at 60%.
2.4 Experimental setup and sampling
A 45-day pot experiment consisting of 36 pots (plant experiment, Brassica) was conducted in the greenhouse. 36 pots included four treatments i.e., control check (CK), WS700, IPSB+SRB, and IPSB-WS+SRB-WS) and three concentrations of Pb+Zn (low (300+250), moderate (900+750) and high (1800+1500) mg/kg) with three replicates. In addition, another experiment of 72 pots treated as above was control group of single Pb and single Zn, only presenting the data of Brassica growth, the activities of SOD, POD and catalase (CAT), as well as malondiald ehyde (MDA) content in supporting materials. The detailed design of 36 pots as follows. CK: for sterilized IPSB and SRB medium without amendments, the volume ratio of medium to the soil was 10%; WS700: 0.6 g of WS700; IPSB+SRB: 2% of IPSB and 2% of SRB (2% is inoculation volume relative to the culture medium) with the same volume ratio as CK; IPSB-WS+SRB-WS: 0.6 g of sterile WS700 was added to a 50 mL anaerobic fermenter and conical flask containing sterile SRB medium and IPSB medium, respectively. Subsequently, 2% of SRB and 2% of IPSB were respectively inoculated into the fermenter and conical flask. The mixture was then incubated in a shaker (120 r/min) at 28 ℃ for 18 h. After incubation, the residual medium was separated from the fermenter and conical flask, mixed together, and then washed three times with 1% normal saline. The finally obtained residual was denoted as IPSB-WS+SRB-WS.
Soil samples (0-20 cm) were collected, ground by 2-mm sieve, and then refrigerated to determine soil Pb and Zn fractions after remediation of 45 d. Brassica samples were harvested, rinsed with deionized water to remove soil particles of roots and shoots, and then refrigerated to analyze physiological properties.
2.5 Sample analysis
Soil pH was detected at a ratio of 1:2.5 (soil: water) [14]. The fractions of Pb and Zn were investigated by Tissier sequential extraction method [15]. Available phosphorus (AP) and potassium (AK) were measured by molybdenum antimony colorimetric method and flame atomic absorption spectrophotometry, respectively [4]. The CEC was measured by saturating the sample with sodium acetate (NaOAc), followed by extraction and analysis [10]. All of Brassica samples from each pot were divided into its shoots and roots parts, which was further divided into two portions. One part was weighed, oven-dried at 105 ℃ for 30 min, and then dried at 70 ℃ until the values of dry shoots and roots were constant. The final fresh and dry weight values were recorded. The other fresh part was powdered using the mortar and pestle to obtain the crude homogenates for determining physiological properties with the appropriate pre-cooled buffer. The pre-cooled buffer, 100 mmol/L phosphate buffer (pH 7.5) for CAT, SOD and POD, 1% (w/V) trichloroacetic acid for MDA, and 3 mL 80% acetone for chlorophyll were prepared to determine activity [16]. Then, the homogenates were centrifuged at 12000 r/min at 4 ℃ for 30 min to obtain supernatants, in which enzyme activity was analyzed using kits from Nanjing JianCheng Bioengineering Institute, China, based on the manufacturer’s instructions [16, 17]. The chlorophyll content in supernatants was measured using spectrophotometric methods [17]. The net photosynthetic rate (Pn), intercellular CO2 concentration (Ci) and stomatal conductance (Gsw) of Brassica leaves were measured using the portable photosynthesis system Li-6400 (Li-Cor Inc., Lincoln, NE, USA) between 8:30 am and 12:00 am on the same day [5]. The dry Brassica portions were acid digested by 30% H2O2 and 65% HNO3 at a ratio of 1:1 (V/V) to analyze the Pb and Zn concentrations in Brassica shoots and roots using atomic absorption spectroscopy (iCE 3000 SERIES DIONEX AS-DV, US) [10].
2.6 Data analysis
EXCEL (2020) and SPSS 26.0 software for experimental data analysis, ORIGIN (2020) for figures drawing, and GGally package in R for correlations analysis were utilized. ANOVA was conducted in SPSS 26.0 software.
3 Results and discussion
3.1 Effect on Pb and Zn immobilization in soil
The fractions of Pb and Zn in soil were clearly impacted by the addition of single biochar, combined A. calcoaceticus with Enterobacter sp. and complex bacteria community loaded biochar amendments (Figure 1). The five fractions could be divided into two main categories: easily labile fractions including exchangeable and carbonate bound states; and recalcitrant fractions including Fe-Mn oxide, organic matter bound, and residual fractions [18]. The increase in easily labile Pb and Zn fractions caused the excessive uptake of plant, subsequently entering the food chain and causing damage to human being [1, 2]. Compared with CK, all treatments reduced the exchangeable Pb and Zn fraction, while increasing the residual state Pb and Zn. The immobilization effect of bacterial loaded biochar on Pb and Zn was much better than that of single biochar and complex bacteria treatments. Complex bacterial loaded biochar declined the exchangeable Pb and Zn fraction by 94.69%-98.37% and 94.55%-99.52%, while increasing the residual state Pb and Zn by 75.50%- 208.58% and 96.71%-110.85%) in comparison to CK, respectively. Our previous researches indicated that the mechanisms of immobilization for Pb and Zn mainly involved in physical adsorption, complexation ion-exchange, and π-π EDA interaction [10, 11]. In general, the Fe-Mn oxide and organic fractions of Pb and Zn exhibited opposite trends under identical treatments and concentration gradients. Three treatments increased the Fe-Mn oxide form of Pb and the organic state of Zn, while simultaneously decreasing the organic form of Pb and Fe-Mn oxide state of Zn. This synchronous interaction suggested that Pb has a strong affinity for Fe-Mn oxide, while Zn exhibits a strong affinity for soil organic matter [18]. Our results showed that complex bacterial loaded biochar may have good application prospects for soil remediation contaminated by Pb and Zn.
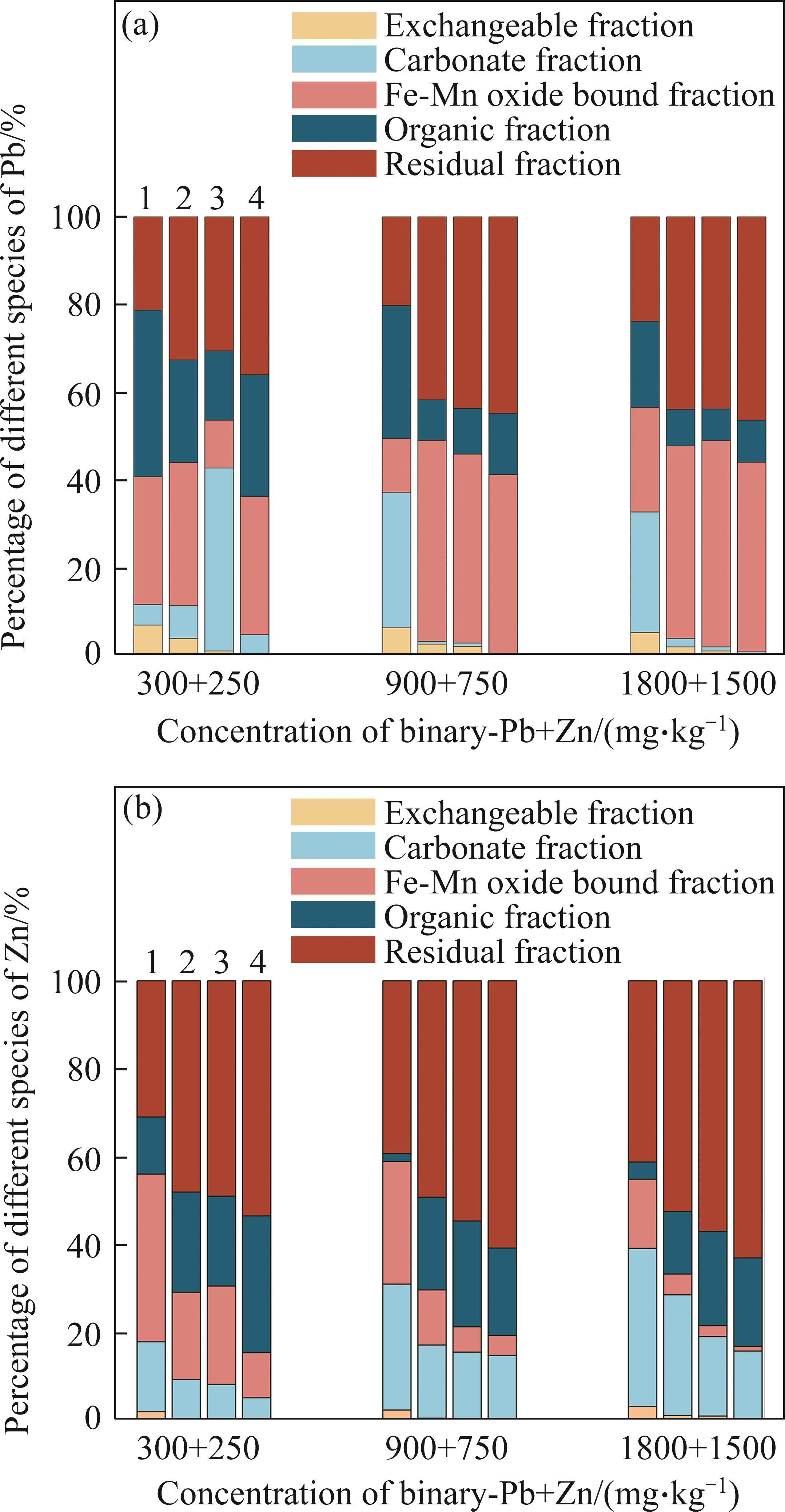
3.2 Effect on Brassica growth
As shown in Figure 2, Pb and Zn co-contamination in soil caused noticeable toxicity to Brassica growth. The fresh and dry biomass of Brassica roots and shoots significantly declined with increasing Pb and Zn concentrations with CK treatment. In addition, the Brassica biomass decreased under pressure from single Zn, complex Pb and Zn and single Pb contamination in the following order: Zn>Pb and Zn>Pb (Figure 2 and Figure S1). An important following hypothesis was that Zn may ameliorate Pb toxicity in Pb and Zn-contaminated soil. SHEN et al [5] indicated that Zn-containing multiple contamination treatments (Zn+Cu, Zn+Pb, Zn+Cu+Pb) could alleviate toxicity of each of them alone existence. Compared with CK, three treatments significantly improved Brassica biomass. When the concentrations of Pb and Zn reached 1800 mg/kg and 1500 mg/kg respectively, the application of WS700, complex bacteria, and complex bacteria loaded biochar led to significant increases in the fresh biomass of Brassica roots by 65.12%, 232.56%, and 408.14%, and shoots by 5.97%, 115.69%, and 180.85% respectively. Compared to CK, WS700, complex bacteria, and complex bacteria loaded biochar resulted in increases in the dry weights of roots by 7.62%, 43.09%, and 105.61%, and shoots by 14.59%, 41.87% and 90.98% respectively, at Pb and Zn concentrations of 300 and 250 mg/kg. This indicated that A. calcoaceticus and Enterobacter sp. loaded biochar effectively reduced labile binary Pb and Zn fractions, thereby ameliorating toxicity to Brassica growth. Consequently, complex bacteria loaded biochar exhibited pronounced effects on promoting Brassica growth in heavily contaminated soil.
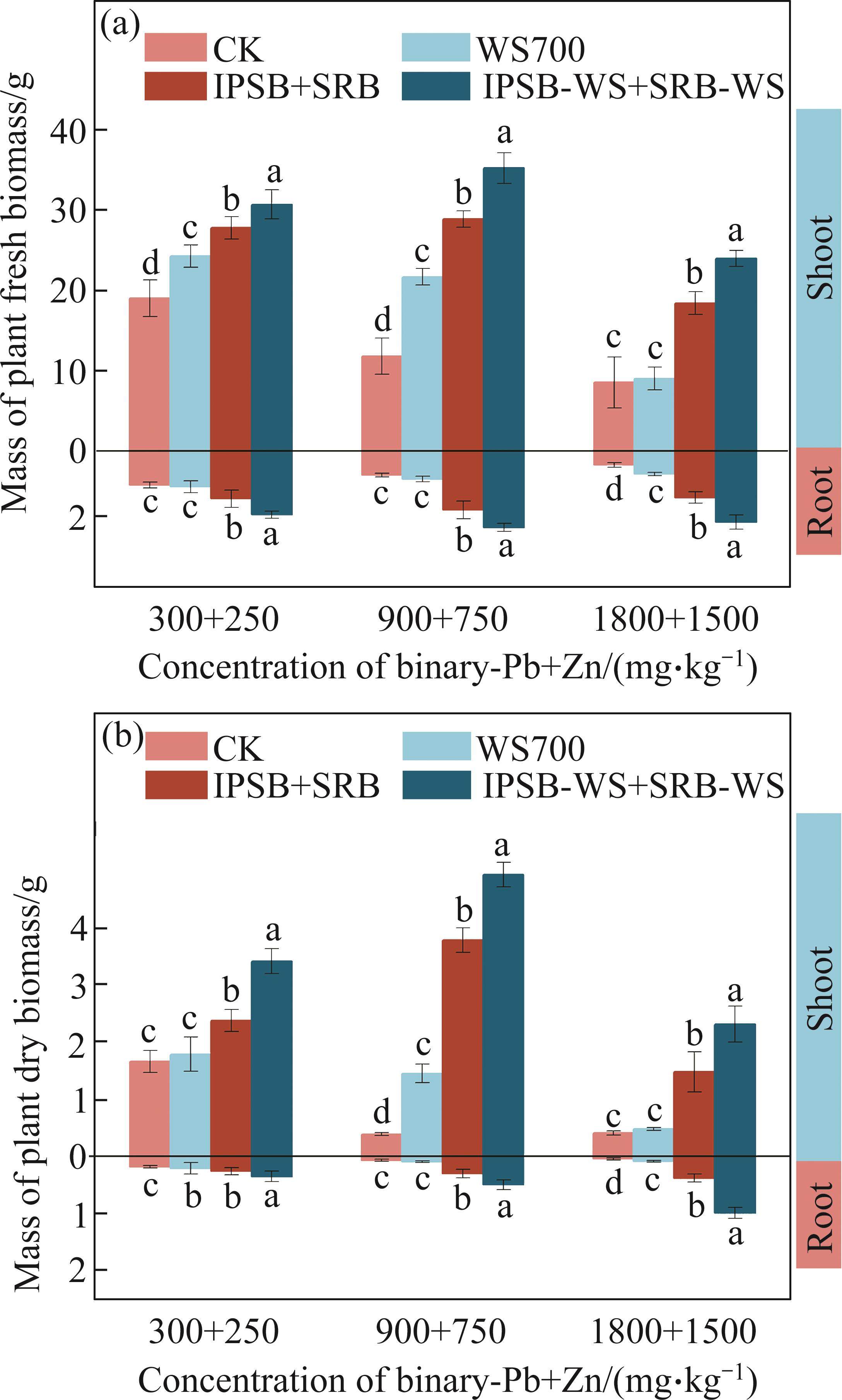
3.3 Effect on photosynthesis characteristics of Brassica
To unveil the mechanisms of three amendments mitigating the toxicity of Pb and Zn to Brassica growth, here, we analyzed total chlorophyll content and photosynthetic characteristics (Figure 3). Brassica from treated soil presented significantly higher total chlorophyll content than that from control soil (Figure 3(a)). Compared with CK, complex bacteria loaded biochar performed the most noticeable effect, enhancing total chlorophyll content by 65.57%-100.63% at the moderate ((900+750) mg/kg) and the high concentration ((1800+1500) mg/kg) of Pb and Zn, which confirmed that bacteria-biochar consortia may alleviate the toxicity effects of Pb and Zn on photosynthetic apparatuses of Brassica. Whereas, the effect of bacteria-biochar consortia on total chlorophyll content was lower than that of other two treatments at the low concentration ((300+250) mg/kg) of Pb and Zn. It may be ascribed as that bacteria-biochar consortia excessively reduced the bioavailable Zn concentration, inhibiting the biosynthesis of chlorophyll content [5]. The result showed that redundant amendment could not be in favor of the biosynthesis of chlorophyll at the low concentration of Pb and Zn. Furthermore, the photosynthetic properties including Pn, Ci and Gsw were compared (Figures 3(a), (b) and (c)). In general, although biochar, A. calcoaceticus and Enterobacter sp. consortia and bacteria consortia loaded biochar increased Pn and Gsw, whilst reducing Ci, there was no significant difference between single biochar and bacteria consortia treatments. When the concentration of Pb and Zn was (900+750) mg/kg, biochar, bacteria consortia and bacteria consortia loaded biochar increased Pn by 10.06%-326.75%, improved Gsw from 0.08 mol/(m2·s), to 1.74, 1.76 and 14.21 mol/(m2·s), and reduced Ci by 1.85%- 14.02%. There were good photosynthesis and chlorophyll responses of Brassica to three treatments, especially bacteria loaded biochar based on the above results. After bacteria-biochar consortia addition, biochar rendered habitats and nutrients to the microorganism, fixing labile Pb and Zn, solubilizing phosphorus and carbon or producing S2- and siderophores to improve the net photosynthetic rate and chlorophyll content of Brassica [17]. Brassica mediated stomatal conductance and reduced intercellular CO2 concentration, enhancing CO2 assimilation into organic matter in response to bacteria-biochar consortia addition, thus alleviating Pb and Zn toxicity. The bacteria-biochar consortia treatment may mitigate interference with the transportation of some nutrients related C, P, Fe, Mn and S elements to leaves, which are essential for chlorophyll synthesis, photosynthetic apparatus and Brassica growth [1, 17].
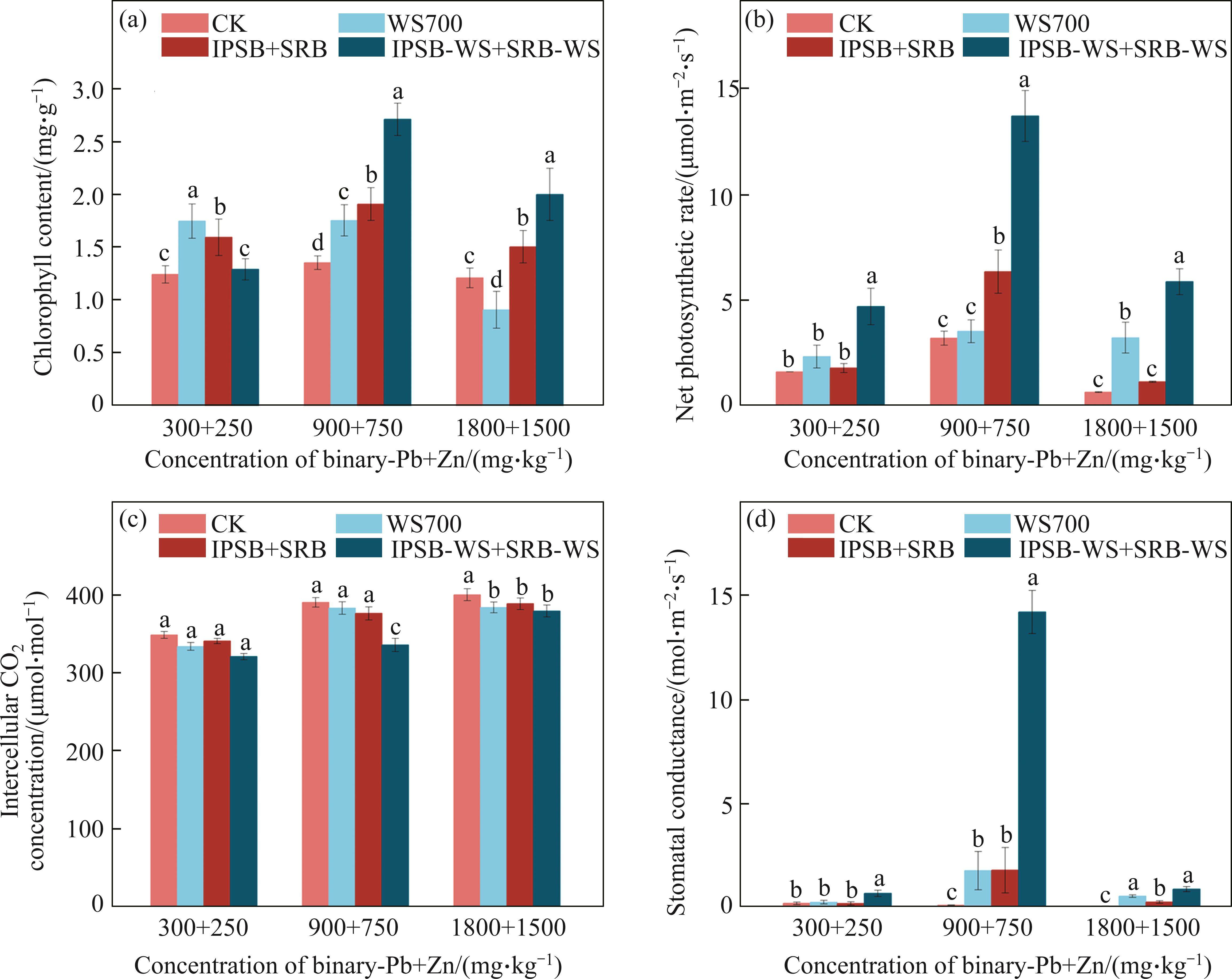
3.4 Effect on antioxidant enzyme activities and MDA content in Brassica
In plants, intrinsic antioxidant enzymes system serves as cleaner of reactive oxygen species (ROS), which mainly includes hydrogen peroxide (H2O2), hydroxyl radical (-OH), superoxide radical (O2-), and singlet oxygen (1O2) to detoxify harmful effects [17, 19]. Pb and Zn could trigger oxidative damages of Brassica, producing excessive ROS in mitochondria, chloroplast and peroxisomes [19, 20]. The activity of the antioxidant enzyme SOD, responsible for converting O2- to H2O2, generally exhibited an increase followed by a decrease under CK treatment as the Pb and Zn concentration increased. This pattern suggested that the response of the antioxidant enzyme system to Pb and Zn stress was constrained (Figure 4(a)). POD could detoxify oxidative damages by scavenging H2O2 into water (H2O) and oxygen molecular (O2), presenting the similar change trend to SOD with CK treatment (Figure 4(b)). The activity of CAT, which further converted excessive H2O2 unscavenged by POD into H2O and O2, increased with the rising Pb and Zn concentration (Figure 4(c)). These results showed that the high concentration of Pb and Zn may break up the dynamic equilibrium between H2O2 synthesis and elimination of enzymatic defense systems [18-20]. Redundant accumulations of ROS may damage the chloroplast and mitochondria membrane and structure, thus suppressing the SOD and POD activities, while simulating CAT activities in peroxisomes [6]. In addition, three treatments increased the activities of SOD and POD except for SOD with single biochar and bacteria consortia treatments, while reducing CAT activities, which was in accordance with the study of IBRAHIM et al [19]. The bacteria-biochar consortia treatment improved SOD and POD activities by 33.99% and 16.45%, respectively, whilst decreasing CAT activities by 80.80% at the high Pb and Zn concentration in comparison with CK. It may be attributed to that the bacteria-biochar consortia treatment declined the bioavailable Pb and Zn fraction and increased SOD and POD activities to eliminate ROS. To maintain the dynamic equilibrium between H2O2 synthesis and elimination, the signal of lower H2O2 reduced CAT activities after the addition of amendments [6]. The results demonstrated that the bacteria-biochar consortia treatment may protect antioxidant enzymes system of Brassica from Pb and Zn toxicity. MDA content represents the levels of lipid peroxidation of plant cell membranes, reflecting the oxidative damages of Brassica. MDA content increased under CK treatment with the increasing Pb and Zn concentration (Figure 4(d)). Meanwhile, MDA content under Pb and Zn stress was lower than that under each of alone stress with CK treatment (Figure 4(d) and Figure S2). Thus, it was reasonable to speculate that moderate Zn may mitigate Pb toxicity in Pb and Zn-contaminated soil. Moreover, compared with CK, three amendments (biochar, bacteria consortia, biochar-bacteria consortia) reduced MDA content by 28.84%, 28.30% and 41.60% at the high concentration of Pb and Zn, respectively. It indicated that three amendments alleviated the damage to cellar proteins, nucleic acids and lipids of Brassica [2].
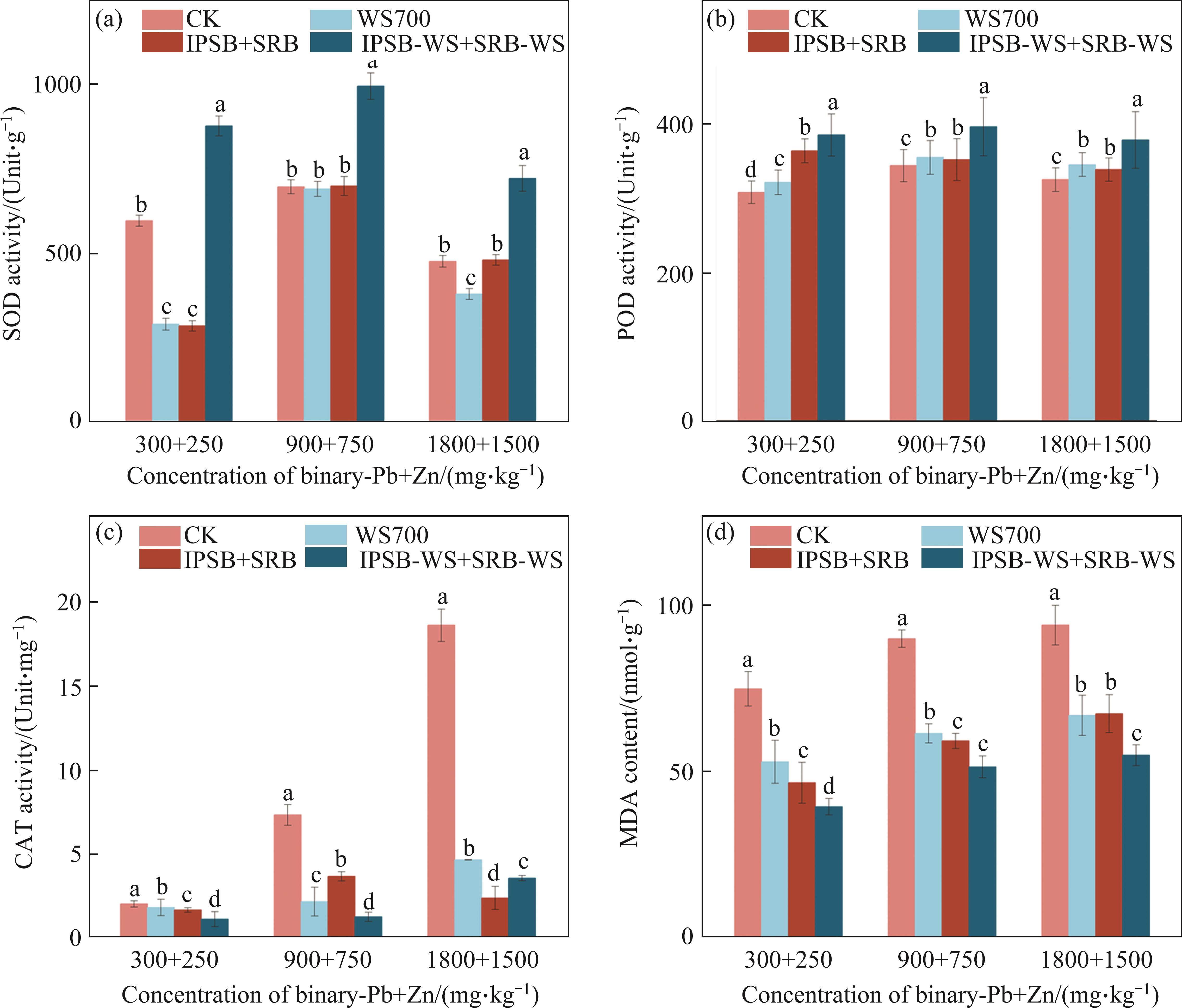
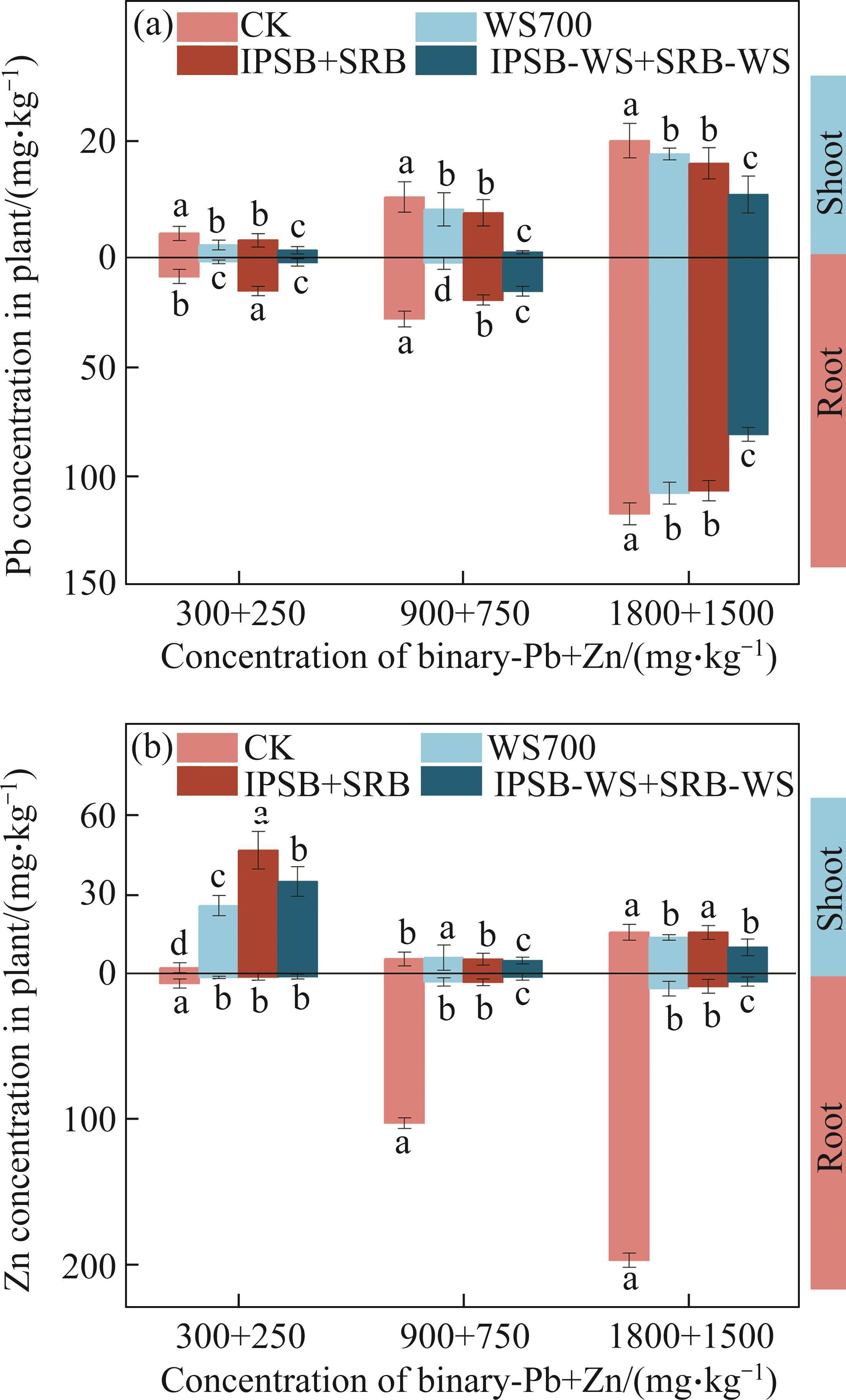
3.5 Effect on Pb and Zn uptake in Brassica
As shown in Figure 5, Pb and Zn mainly distributed in Brassica roots rather than in shoots, and the concentration of Zn in Brassica shoots was generally three times greater than that of Pb in shoots at low concertration ((300+250) mg/kg), which may be attributed that Zn are plant essential micronutrients, but Pb not [5]. Compared with CK, three amendments (biochar, bacteria consortia, biochar-bacteria consortia) declined the concentration of Pb in Brassica roots by 8.11%, 9.06% and 30.98%, and reduced that of Zn by 94.68%, 95.45% and 97.10% at the high concentration of Pb and Zn, respectively. The results presented that A. calcoaceticus and Enterobacter sp. colonization and biochar addition had a significant effect on the Pb and Zn uptake and distribution in Brassica plants.
3.6 Interactive effects of Pb and Zn on physiological responses of Brassica
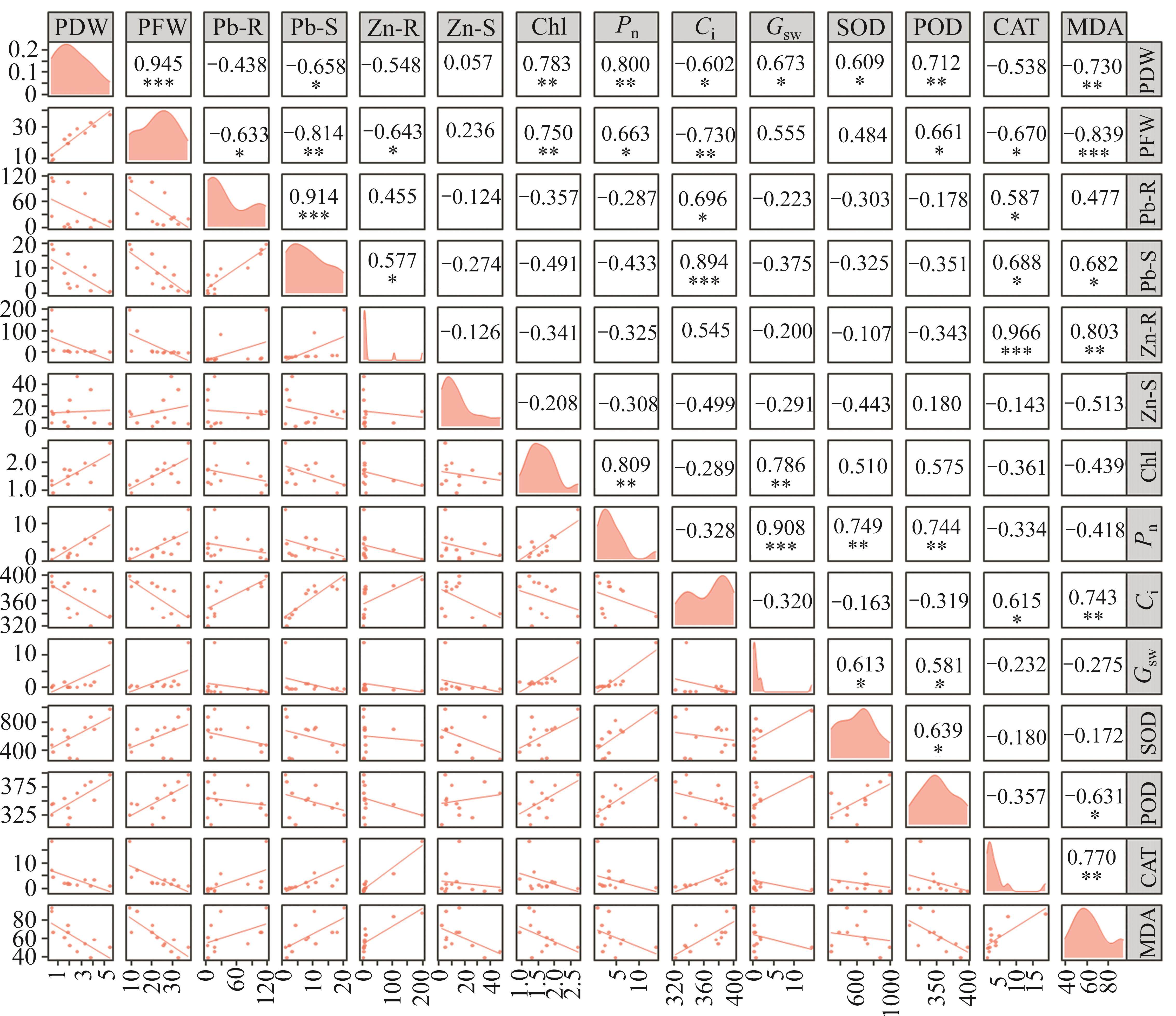
The concentration of Pb in shoots of Brassica was significantly (p<0.001) increased with the increasing in root. However, the concentration of Zn in shoot and root was not significantly correlated (Figure 6), which may result from essential Zn and non-essential Pb belonging to differently alternative translocation pathway. In addition, the fresh and dry biomass had a significantly negative effects on Pb in shoots and roots, whereas they had no significant effects on Zn. Pairwise comparisons of chlorophyll content, net photosynthetic rate and stomatal conductance positively correlated with each other (p<0.01). SOD had a significantly positive effect (p<0.05) on POD, and they significantly improved the fresh and dry weights of Brassica (p<0.05). CAT positively correlated with MDA, as reported that elevating oxidative damages stimulated CAT activities in plant cells [19, 21, 22].
4 Conclusions
The present study demonstrated that biochar, IPSB and SRB consortia, and the consortia loaded biochar, effectively immobilized the exchangeable Pb and Zn fraction by 94.69%- 98.37% and 94.55%-99.52%. Moreover, three treatments simultaneously increased the Fe-Mn oxide-bound Pb and organic Zn fractions while decreasing the organic Pb and Fe-Mn oxide-bound Zn fractions, suggesting a strong affinity between Pb and Fe-Mn oxides and between Zn and soil organic matter. Additionally, when Pb and Zn concentrations were 1800 and 1500 mg/kg respectively, the consortia-loaded biochar increased fresh root and shoot biomass by 408.14% and 180.85%, respectively. Brassica responded to the addition of bacteria-biochar consortia by mediating stomatal conductance and reducing intercellular CO2 concentration, thus enhancing CO2 transformation into organic matter and alleviating Pb and Zn toxicity. Compared with CK, bacteria-biochar consortia treatment improved total chlorophyll content by 100.63% under moderate Pb and Zn concentrations. Moreover, consortia-loaded biochar treatment improved SOD and POD activities by 33.99% and 16.45% respectively, while decreasing CAT activities and MDA content by 80.80% and 28.84% respectively, at high Pb and Zn concentrations compared to CK. Furthermore, compared to CK, the three amendments reduced Pb concentration in Brassica roots by 8.11%, 9.06%, and 30.98%, and Zn concentration by 94.68%, 95.45% and 97.10%, respectively, at high Pb and Zn concentrations. In summary, consortia-loaded biochar plays a crucial role in alleviating the toxic effects of Pb and Zn on Brassica’s photosynthetic apparatus and antioxidant enzyme system. Our results are significant for understanding the mechanisms that connect soil health, plant growth, and soil functioning resilience, which are essential for effectively and sustainably remediating Pb and Zn contamination using consortia-loaded biochar.
Barley yield and soil microbial and enzyme activities as affected by contamination of two soils with lead, zinc or copper
[J]. Biology and Fertility of Soils, 2005, 41(2): 85-94. DOI: 10.1007/s00374-004-0820-9.Field assessment of carboxymethyl cellulose bridged chlorapatite microparticles for immobilization of lead in soil: Effectiveness, long-term stability, and mechanism
[J]. Science of the Total Environment, 2021, 781: 146757. DOI: 10.1016/j.scitotenv.2021.146757.Ecological evaluation of heavy metal pollution in the soil of Pb-Zn mines
[J]. Ecotoxicology, 2022, 31(2): 259-270. DOI: 10.1007/s10646-021-02505-3.Effect of potentially toxic elements on soil multifunctionality at a lead smelting site
[J]. Journal of Hazardous Materials, 2023, 454: 131525. DOI: 10.1016/j.jhazmat.2023.131525.Interactive effects of single, binary and trinary trace metals (lead, zinc and copper) on the physiological responses of Kandelia obovata seedlings
[J]. Environmental Geochemistry and Health, 2019, 41(1): 135-148. DOI: 10.1007/s10653-018-0142-8.Plant peroxisomes: Recent discoveries in functional complexity, organelle homeostasis, and morphological dynamics
[J]. Current Opinion in Plant Biology, 2016, 34: 17-26. DOI: 10.1016/j.pbi.2016.07.008.Litsea males are better adapted to Pb stress than females by modulating photosynthesis and Pb subcellular distribution
[J]. Forests, 2023, 14(4): 724. DOI: 10.3390/f14040724.Bioformulation of biochar as a potential inoculant carrier for sustainable agriculture
[J]. Environmental Technology & Innovation, 2020, 20: 101168. DOI: 10.1016/j.eti.2020.10 1168.Biochar-bacillus consortium for a sustainable agriculture: Physicochemical and soil stability analyses
[J]. Biochar, 2023, 5(1): 17. DOI: 10.1007/s42773-023-00215-z.Bioremediation of lead-contaminated soil by inorganic phosphate-solubilizing bacteria immobilized on biochar
[J]. Ecotoxicology and Environmental Safety, 2022, 237: 113524. DOI: 10.1016/j.ecoenv.2022.113524.Immobilized sulfate reducing bacteria (SRB) enhanced passivation performance of biochar for Zn
[J]. Science of the Total Environment, 2023, 892: 164556. DOI: 10.1016/j.scitotenv. 2023.164556.Effective passivation of lead by phosphate solubilizing bacteria capsules containing tricalcium phosphate
[J]. Journal of Hazardous Materials, 2020, 397: 122754. DOI: 10.1016/j.jhazmat.2020.122754.Enhanced bioremediation of heavy metal from effluent by sulfate-reducing bacteria with copper–iron bimetallic particles support
[J]. Bioresource Technology, 2013, 136: 413-417. DOI: 10.1016/j.biortech.2013.03.047.Study on soil physical structure after the bioremediation of Pb pollution using microbial-induced carbonate precipitation methodology
[J]. Journal of Hazardous Materials, 2021, 411: 125103. DOI: 10.1016/j.jhazmat.2021.125103.Sequential extraction procedure for the speciation of particulate trace metals
[J]. Analytical Chemistry, 1979, 51(7): 844-851. DOI: 10.1021/ac50043a017.Acidic amelioration of soil amendments improves soil health by impacting rhizosphere microbial assemblies
[J]. Soil Biology and Biochemistry, 2022, 167: 108599. DOI: 10.1016/j.soilbio.2022.108599.Antimony-oxidizing bacteria alleviate Sb stress in Arabidopsis by attenuating Sb toxicity and reducing Sb uptake
[J]. Plant and Soil, 2020, 452(1): 397-412. DOI: 10.1007/s11104-020-04569-2.Biochar and bacteria inoculated biochar enhanced Cd and Cu immobilization and enzymatic activity in a polluted soil
[J]. Environment International, 2020, 137: 105576. DOI: 10.1016/j.envint. 2020.105576.Biochars effects potentially toxic elements and antioxidant enzymes in Lactuca sativa L. grown in multi-metals contaminated soil
[J]. Environmental Technology & Innovation, 2019, 15: 100427. DOI: 10.1016/j.eti.2019.100427.Arbuscular mycorrhizal fungal inoculation protects Miscanthus× giganteus against trace element toxicity in a highly metal-contaminated site
[J]. Science of the Total Environment, 2015, 527-528: 91-99. DOI: 10.1016/j.scitotenv.2015. 04.116.Heavy metal(loid)s transformation in dust at a lead smelting site
[J]. Journal of Central South University, 2024, 31(4): 1036-1049. DOI: 10.1007/s11771-024-5600-0.Soil microbial community in lead smelting area and the role of sulfur-oxidizing bacteria
[J]. Journal of Central South University, 2024, 31(4): 1050-1063. DOI: 10.1007/s11771-024-5601-z.LI Xue, ZHU Xiao-li, ZHU Feng, LI Xing, ZHANG Zi-ye and XUE Sheng-guo declare that they have no conflict of interest.
LI Xue, ZHU Xiao-li, ZHU Feng, LI Xing, ZHANG Zi-ye, XUE Sheng-guo. Interactive effects of bacteria-loaded biochar on the physiological responses of Brassica rapa var. chinensis in the Pb and Zn contaminated soil [J]. Journal of Central South University, 2025, 32(1): 149-159. DOI: https://doi.org/10.1007/s11771-025-5848-z.
李雪,朱晓丽,朱锋等.细菌胶团和生物炭联合修复对铅性复合污染土壤的小青菜生理作用效应[J].中南大学学报(英文版),2025,32(1):149-159.

