脑梗死即缺血性脑卒中,是最常见的卒中类型。脑梗死是多种原因导致脑部血流供应减少或血管闭塞进而引发的的脑血管疾病,临床上常表现为血流闭塞区域相关神经功能损伤,并出现相应神经系统症状。相较于脑出血而言,脑梗死的生存率稍高,但更易导致神经系统相关功能缺损[1],不仅会造成肢体残疾,还会导致认知障碍、抑郁等精神症状[2]。
认知功能障碍是脑梗死常见的并发症,临床上表现为记忆、言语、执行、计算和理解等某一认知域或多认知域的损害[3]。患者认知功能障碍表现与梗死涉及的具体部位区域和范围大小有关。当梗死部位位于海马时,患者的空间定向、情景记忆、知识的学习和储存等可出现障碍[4];当梗死部位位于前额叶皮层时,患者的记忆、执行能力、注意力等认知功能会出现缺损[5-6];此外,内侧前额叶皮层发生梗死导致的认知障碍的病程较长[7]。随着时间的推移,脑梗死引起的认知障碍或逐渐好转,或缓慢进展,甚至发展为痴呆。其进展严重程度受多因素影响,包括患者的年龄、脑梗死起病时间、部位、梗死面积、神经元损伤、侧支循环的情况和其他大脑功能障碍[8]。目前,脑梗死相关认知障碍的相关机制复杂且不明确,给该病的诊疗带来了难题。从周细胞变性、活性氧(reactive oxygen species,ROS)生成过量、谷氨酸生成过量、自噬过度激活等4个方面概述脑梗死后认知障碍的相关机制,旨在为脑梗死相关认知障碍的治疗提供新的思路。
周细胞变性
在生理状态下,周细胞的主要生理功能包括脑血流量(cerebral blood flow,CBF)的调控、血脑屏障(blood-brain barrier,BBB)的维持、血管生成、瘢痕的形成及炎症细胞的运输5个方面[9]。当脑梗死发生后,周细胞收缩变性、功能出现异常,挤压脑微血管,使微血管长期处于收缩状态,大脑持续处于缺血/缺氧状态,进一步加重脑损伤[10],导致认知障碍发生[6]。
周细胞是激活神经炎症的重要介质。一方面,周细胞能够促使神经胶质细胞转变为促炎表型,分泌一系列炎症相关细胞因子,如白细胞介素(interleukin,IL)-1β、IL-6和肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)等,引发强烈的炎症级联反应[11-12],炎症因子破坏BBB,促使炎性介质通过主动运输(直接)或通过迷走神经刺激(间接)进入大脑,脑白质纤维结构破坏及胆碱能通路损害,信息传递出现阻碍,致使认知障碍的发生[13]。Duan等[14]的研究也证实了这一观点。另一方面,周细胞表达的细胞间黏附分子-1(intercellular cell adhesion molecule-1,ICAM-1)与白细胞上的整合素配体相互作用,募集白细胞完成炎性介质的迁移,将炎性信号传递给神经元,导致神经元之间相互缠结,突触功能障碍,最终导致认知障碍[15](图1)。
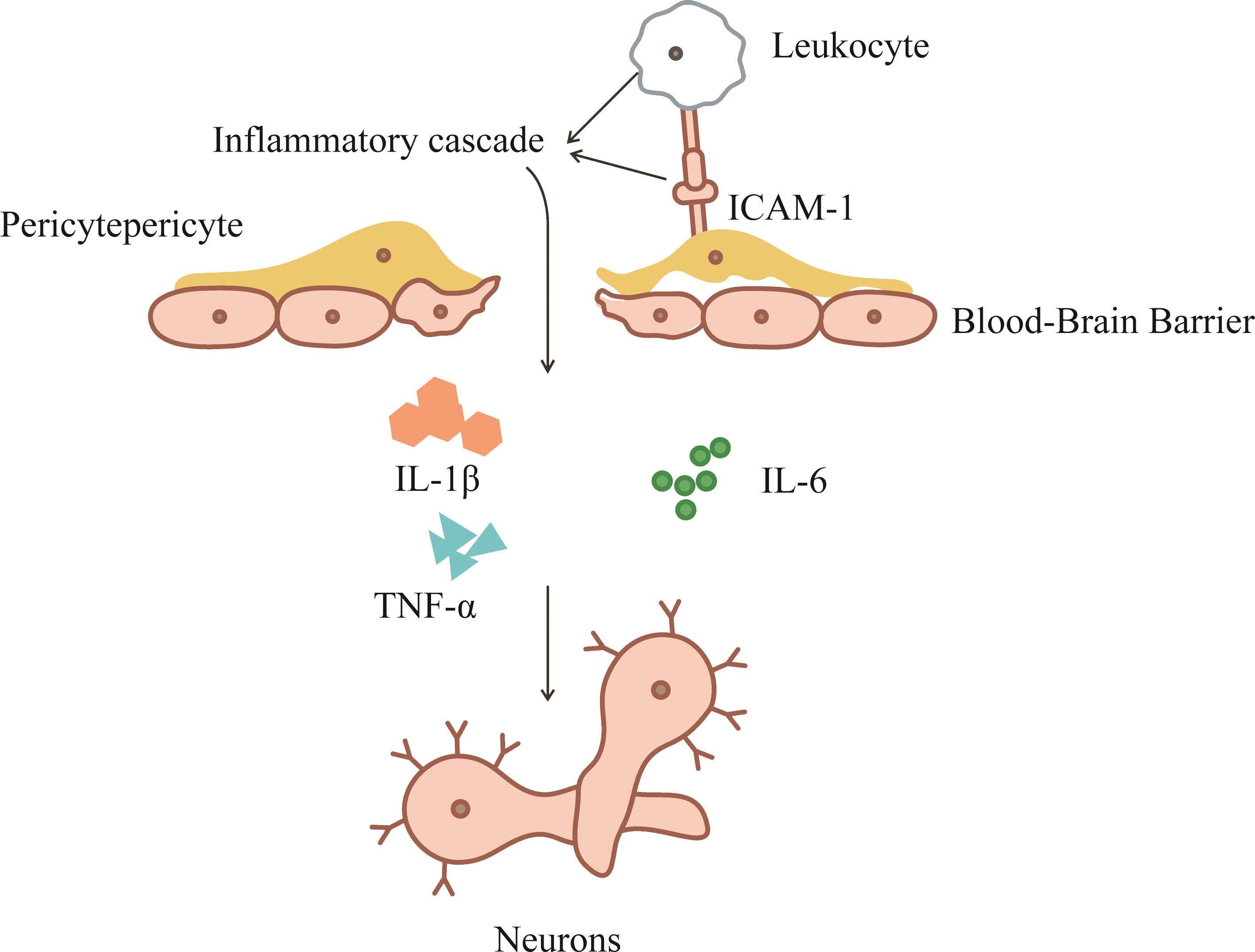
Hesp等[16]发现:脑梗死发生后周细胞脱离毛细血管,降解紧密连接蛋白(tight junctions proteins,TJs)和黏附连接蛋白(adherens junctions proteins,Ajs),破坏内皮细胞间的紧密连接,导致BBB完整性被破坏,β-淀粉样蛋白(amyloid β-protein,Aβ)清除率降低并异常沉积在脑实质中,影响突触传递,甚至加重炎症级联反应,进一步加重认知障碍的发展[17]。Liu等[18]通过对72例脑梗死/短暂性脑缺血发作后出现认知障碍的患者追踪随访发现Aβ的沉积明显加重并加速认知衰退的进程。Zlokovic等[19]也提出了类似的观点。
周细胞通过促进血管生成和瘢痕形成减轻脑损伤,缓解脑梗死造成的认知障碍。在脑梗死状态下,周细胞被募集并迁移至脑梗死区域,不仅参与血管生成,缓解了梗死周围区域的缺血状态,还在梗死与非梗死区域之间形成胶质瘢痕,防止神经元进一步的丢失[20]。虽然胶质瘢痕的形成有助于防止脑损伤的进一步加重,但胶质瘢痕过度形成会抑制轴突再生,阻碍神经元的恢复[21]。
ROS生成过量
ROS生成过量是脑梗死发生认知障碍的重要因素[22-23]。当患者出现脑梗死后,其大脑的局部缺血组织处于缺血/缺氧状态,促使脑组织产生大量的ROS[24],当ROS产生的量超过机体内源性抗氧化剂和抗氧化酶的保护清除能力时,会激活氧化应激反应[25]。动物实验[23]发现氧化应激与认知功能障碍的发生有关。Zhang等[22]的研究也同样证明神经保护剂通过抑制氧化应激来改善认知功能。一方面,氧化应激会导致线粒体功能障碍[26]和蛋白质错误折叠沉积[27],影响神经元完整性和基因表达,进而通过引起海马树突结构的变化影响认知能力[28]。当线粒体功能出现障碍时,过量的ROS产生、钙超载、细胞色素C释放和凋亡通路激活等一系列异常的生理过程也会随之出现,从而导致DNA损伤和caspase依赖性凋亡细胞死亡,进而进一步加重脑损伤[29-30](图2)。另一方面,氧化应激会介导细胞铁依赖性的死亡,即铁死亡[31]。研究[32]表明,在氧葡萄糖剥夺/再灌注(oxygen-glucose deprivation/reoxygenation,OGD/R)诱导的神经元损伤的模型中,抑制铁死亡可以改善神经元的损伤及脑梗死相关的认知障碍。此外,铁死亡过程也会通过介导过渡金属铁的酶促反应产生可溶性和脂质ROS[33],而过量的ROS可通过过度氧化脂质、蛋白质、破坏DNA等方式破坏生物分子的结构和功能,从而导致细胞坏死、神经元损伤,最终导致认知障碍[34]。此外,氧化应激通过抑制脑血管内皮细胞中的内皮型一氧化氮合酶/一氧化氮(endothelial nitric oxide synthase/nitric oxide,eNOS/NO)信号,阻碍脑源性神经营养因子的合成,从而抑制突触可塑性和神经再生,机体记忆和大脑学习能力的下降,最终导致认知障碍的发生[35]。
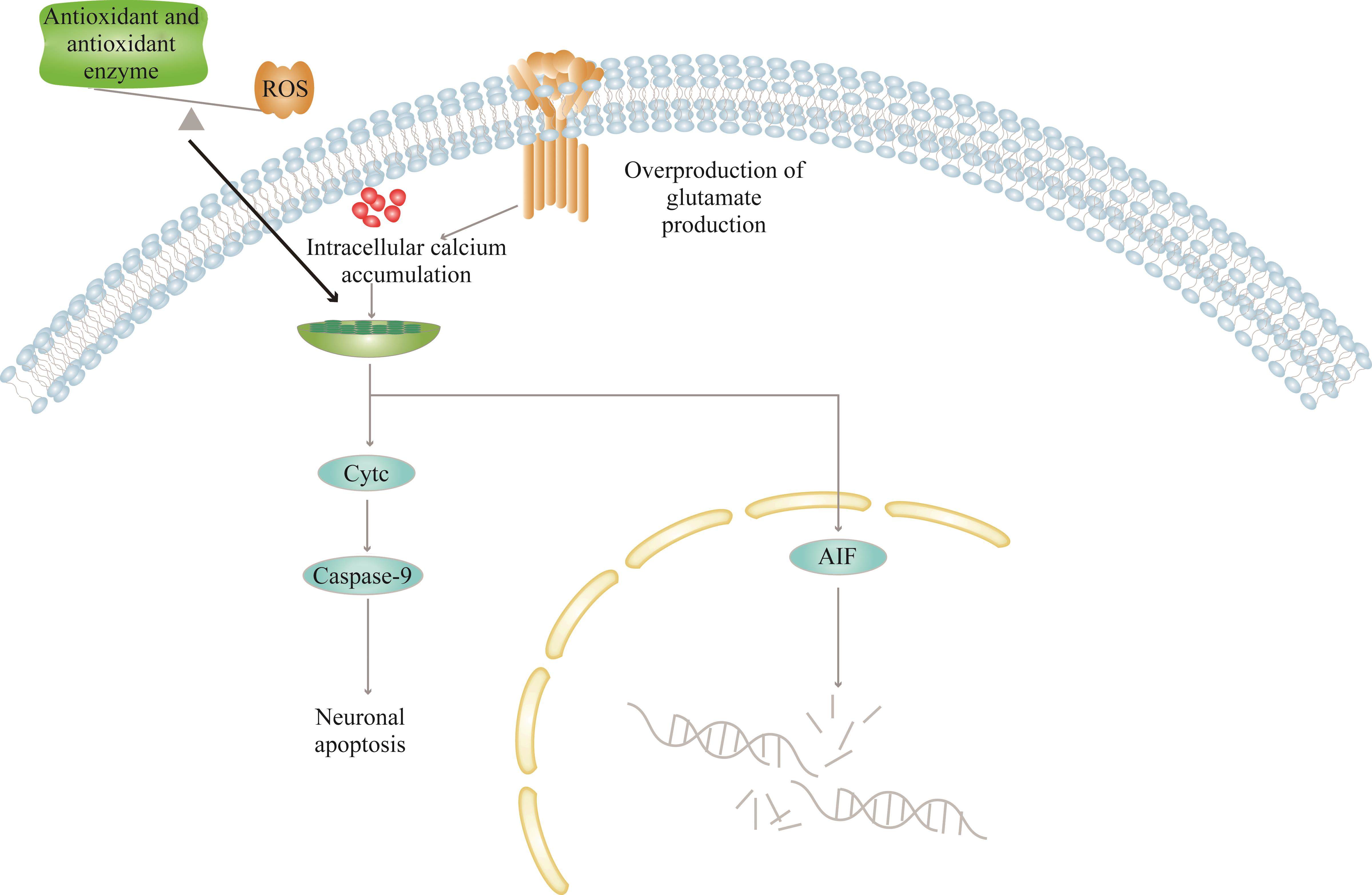
在脑梗死发生后,体内内源性抗氧化剂如谷胱甘肽和抗氧化酶如谷胱甘肽过氧化物酶(glutathione peroxidase,GPX)、超氧化物歧化酶(superoxide dismutase,SOD)[36]、过氧化氢酶等[34]被耗尽,抗氧化相关因子也被下调,从而导致认知障碍。研究发现,增强抗氧化相关因子如核因子E2相关因子2(nuclear factor erythroid 2-related factor 2,Nrf2)等可以减轻ROS对认知能力的损伤,保护神经元活性[37],从而改善脑梗死相关的认知障碍[38]。
谷氨酸生成过量
谷氨酸是中枢神经系统中重要的兴奋性神经递质。在正常生理条件下,谷氨酸的产生和清除处于动态平衡,以维持细胞外液中谷氨酸的较低浓度水平。在脑梗死的病理条件下,由于氧和葡萄糖的大量消耗,释放谷氨酸的量远远大于清除的量。过量的谷氨酸积累在细胞间隙中,不能被能量依赖性突触前突触重新摄取[39],因此神经元和神经胶质细胞上的N-甲基-D-天[门]冬氨酸(N-methyl-D-aspartate,NMDA)受体[40]、α-氨基-3-羟基-5-甲基-4-异唑(α-amino-3-hydroxy-5-methyl-4-isox-azolepropionic acid,AMPA)受体被过度刺激[41],继而出现一系列继发的神经损伤,即兴奋性毒性。受体过度刺激引发神经元内Ca2+内流[42],一方面,激活蛋白激酶和核酸内切酶,导致神经细胞中核酸、蛋白质代谢等重要物质降解,使得神经元变性或死亡;另一方面,激活并增强Ca2+依赖的凋亡途径,进一步促使神经元死亡,脑组织萎缩,出现认知功能下降(图2)。此外,也有研究[43]表明这种兴奋性毒性导致白质疏松症等脑部病理变化,最终导致认知功能障碍。
自噬过度激活
在正常的生理活动中,自噬是一种机体自我保护的过程。大脑缺血/缺氧时,神经元的自噬过程在脑梗死中起双重作用。研究[44-45]表明:大脑缺血/缺氧时,自噬被过度激活,破坏海马神经元的自噬通量,导致溶酶体膜通透性改变及溶酶体腔内酶外渗,自噬小体无法与溶酶体结合进行底物降解,加重脑损伤,出现认知障碍(图3)。而当自噬被抑制就可以起到神经保护的作用。在缺血/缺氧性脑损伤模型的新生大鼠中,微管相关蛋白1轻链3-II(microtubule-associated protein 1 light chain 3-II,LC3-II)表达增加,提示缺血/缺氧诱导神经元过度自噬,进一步加重脑损伤并在青春期诱发认知和记忆障碍。而当新生大鼠大脑中注入自噬抑制剂3-甲基腺嘌呤(3-Methyladenine,3-MA)后,LC3-II的表达被减弱,改善了神经元死亡,减轻了脑损伤[46]。
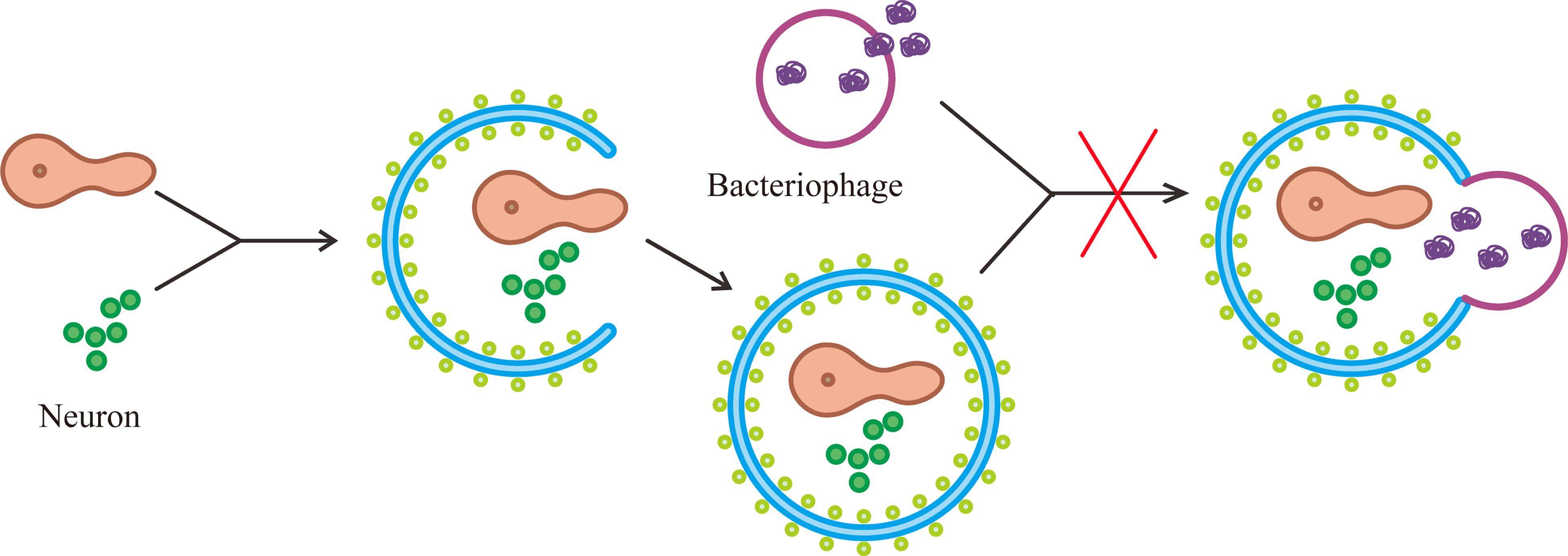
脑梗死发生时,大脑中的其他细胞和细胞器也会发生自噬,加重或减轻认知障碍的程度[47]。在正常的生理情况下,少突胶质细胞维持神经元轴突和神经元功能的完整性[48]。而当大脑缺血/缺氧过度激活自噬时,少突胶质细胞坏死,引起严重脱髓鞘,导致神经冲动传导减慢,记忆和认知形成的通路受阻,最终出现认知功能障碍[49]。反之,线粒体损伤会加重缺血/缺氧导致的脑损伤,因此激活线粒体自噬可以起保护神经的作用,改善认知障碍。线粒体自噬不仅能降解受损的线粒体,还能防止由线粒体DNA突变引起的细胞损伤[50]。Fan等[51]的研究表明,在脑梗死模型大鼠中,RGD1564534通过竞争性结合miR-101a-3p上调DUSP1的表达,促进受损线粒体自噬并减弱NOD样受体热蛋白结构域相关蛋白3(NOD-like receptor thermal protein domain associated protein 3,NLRP3)炎症小体活性,从而缓解脑白质和小血管损伤,改善大鼠的认知功能障碍。Zhong等[52]的研究中通过调节脑梗死状态下大鼠的褪黑素也能得出此结论。
结 语
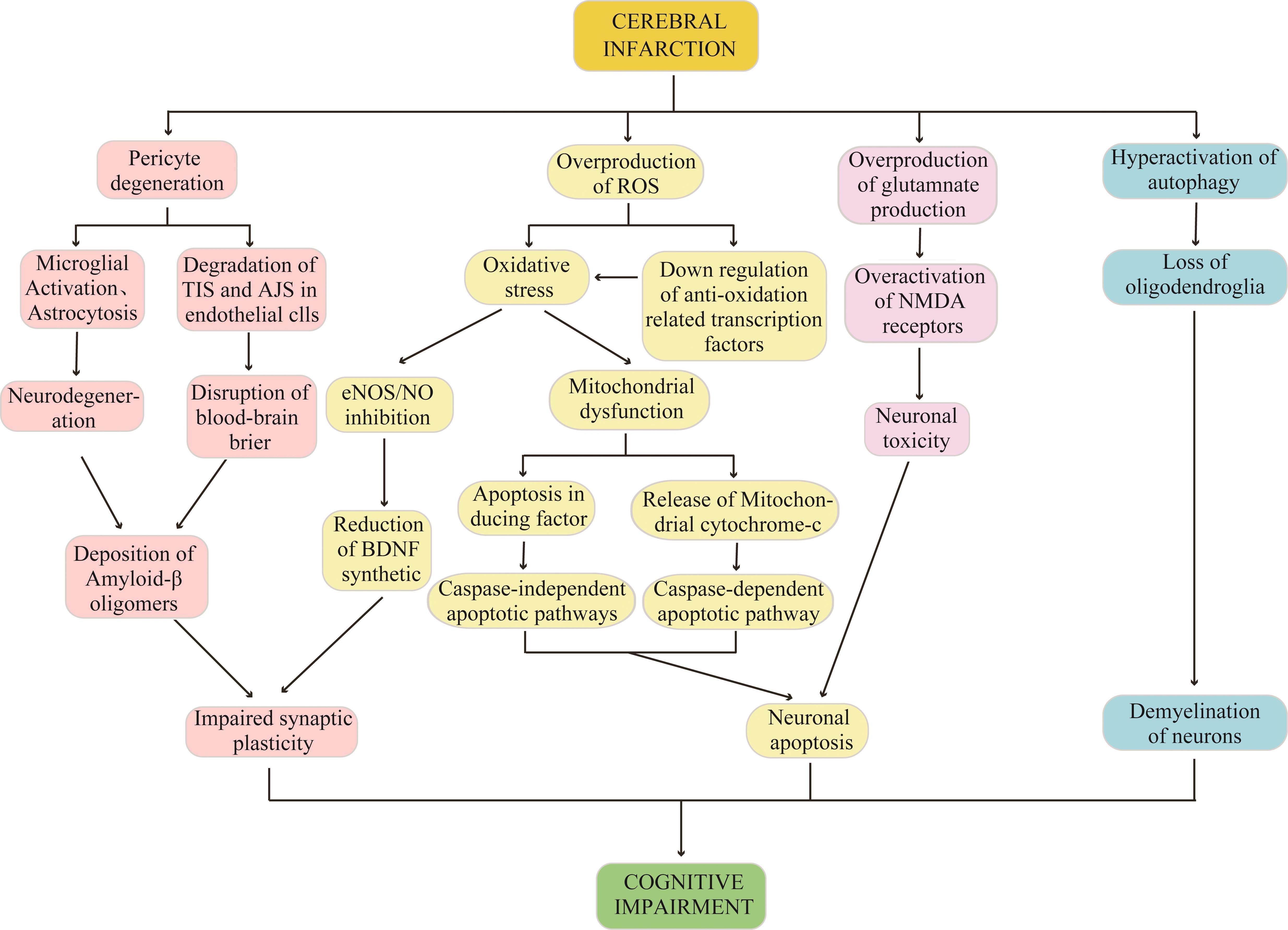
脑梗死患者常合并认知功能障碍。当脑梗死相关的认知障碍与周细胞变性、ROS生成过量、谷氨酸生成过量、自噬过度激活4种病理生理改变有关(图4),但该疾病致病的根本机制仍不明确。现有研究主要证明了某种机制与疾病发生发展之间存在关联,但这些机制间的因果关系尚不清楚。换而言之,氧化应激、兴奋性毒性、自噬过度激活、周细胞变性都是可能导致该疾病和/或其进展的潜在因素。所以,为了充分明确脑梗死相关认知障碍的精确机制及各因素间的因果关系,未来无疑需要进行更多研究。
Early psychiatric morbidity in a Brazilian sample of acute ischemic stroke patients
[J/OL]. Clinics, 2018, 73:Post-stroke cognitive impairment and dementia
[J]. Circulation research, 2022, 130, 8: 1252-1271. https://doi.org/10.1161/CIRCRESAHA.122.319951.Pathophysiology, assessment, and management of post-stroke cognitive impairment, depression, and fatigue
[J]. Phys Med Rehabil Clin N Am, 2024, 35(2): 463-478. https://doi.org/10.1016/j.pmr.2023.06.028.Molecular factors mediating neural cell plasticity changes in dementia brain diseases
[J]. Neural Plast, 2021, 2021: 8834645. https://doi.org/10. 1155/2021/8834645.Updating stored memory requires adult hippocampal neurogenesis
[J]. Sci Rep, 2015, 5: 13993. https://doi.org/10.1038/srep13993.Roles of pericytes in stroke pathogenesis
[J]. Cell Transplant, 2018, 27(12): 1798-1808. https://doi.org/10. 1177/0963689718768455.Assessing cognitive function following medial prefrontal stroke in the rat
[J]. Behav Brain Res, 2015, 294: 102-110. https://doi. org/10.1016/j.bbr.2015.07.053.Longitudinal effect of stroke on cognition: a systematic review
[J/OL]. J Am Heart Assoc, 2018, 7(2): e006443[A novel sensitive assay for detection of a biomarker of pericyte injury in cerebrospinal fluid
[J]. Alzheimers Dement, 2020, 16(6): 821-830. https://doi.org/10.1002/alz.12061.No-reflow phenomenon in the heart and brain
[J]. Am J Physiol Heart Circ Physiol, 2018, 315(3): H550-H562. https://doi.org/10.1152/ajpheart.00183.2018.The role of microglia and myeloid immune cells in acute cerebral ischemia
[J]. Front Cell Neurosci, 2015, 8: 461. https://doi.org/10.3389/fncel.2014.00461.TNF-α-sensitive brain pericytes activate microglia by releasing IL-6 through cooperation between IκB-NFκB and JAK-STAT3 pathways
[J]. Brain Res, 2018, 1692: 34-44. https://doi.org/10.1016/j.brainres. 2018.04.023.White matter degeneration in vascular and other ageing-related dementias
[J]. J Neurochem, 2018, 144(5): 617-633. https://doi.org/10.1111/jnc.14271.PDGFRβ cells rapidly relay inflammatory signal from the circulatory system to neurons via chemokine CCL2
[J]. Neuron, 2018, 100(1): 183-200.e8. https://doi.org/10.1016/j.neuron.2018.08.030.GJ-4 ameliorates memory impairment in focal cerebral ischemia/reperfusion of rats via inhibiting JAK2/STAT1-mediated neuroinflammation
[J]. J Ethnopharmacol, 2021, 267: 113491. https://doi.org/10.1016/j.jep.2020.113491.Cognitive decline according to amyloid uptake in patients with poststroke cognitive impairment
[J/OL]. Medicine, 2021, 100(38): e27252[Influence of amyloid-β on cognitive decline after stroke/transient ischemic attack: three-year longitudinal study
[J]. Stroke, 2015, 46(11): 3074-3080. https://doi.org/10.1161/STROKEAHA.115.010449.Perivascular microglia promote blood vessel disintegration in the ischemic penumbra
[J]. Acta Neuropathol, 2015, 129(2): 279-295. https://doi.org/10. 1007/s00401-014-1372-1.Proliferating NG2-cell-dependent angiogenesis and scar formation alter axon growth and functional recovery after spinal cord injury in mice
[J]. J Neurosci, 2018, 38(6): 1366-1382. https://doi.org/10. 1523/JNEUROSCI.3953-16.2017.Reducing pericyte-derived scarring promotes recovery after spinal cord injury
[J]. Cell, 2018, 173(1): 153-165.e22. https://doi.org/10.1016/j.cell.2018. 02.004.DL-3-n-butylphthalide (NBP) alleviates poststroke cognitive impairment (PSCI) by suppressing neuroinflammation and oxidative stress
[J]. Front Pharmacol, 2023, 13: 987293. https://doi.org/10.3389/fphar.2022. 987293.Enriched environment improves post-stroke cognitive impairment and inhibits neuroinflammation and oxidative stress by activating Nrf2-ARE pathway
[J]. Int J Neurosci, 2021, 131(7): 641-649. https://doi. org/10.1080/00207454.2020.1797722.Therapeutic potential of remote ischemic conditioning in vascular cognitive impairment
[J]. Front Cell Neurosci, 2021, 15: 706759. https://doi.org/10. 3389/fncel.2021.706759.Oxidative stress, antioxidants and intestinal calcium absorption
[J]. World J Gastroenterol, 2017, 23(16): 2841-2853. https://doi.org/10. 3748/wjg.v23.i16.2841.Surface-tailored nanoplatform for the diagnosis and management of stroke: current strategies and future outlook
[J]. Mol Neurobiol, 2024, 61(3): 1383-1403. https://doi.org/10.1007/s12035-023-03635-x.A molecular web: endoplasmic reticulum stress, inflammation, and oxidative stress
[J]. Front Cell Neurosci, 2014, 8: 213. https://doi.org/10. 3389/fncel.2014.00213.Post-stroke cognitive impairment and synaptic plasticity: a review about the mechanisms and Chinese herbal drugs strategies
[J]. Front Neurosci, 2023, 17: 1123817. https://doi.org/10.3389/fnins.2023.1123817.Ischemic stroke and mitochondria: mechanisms and targets
[J]. Protoplasma, 2020, 257(2): 335-343. https://doi.org/10.1007/s00709-019-01439-2.Mitochondria- and oxidative stress-targeting substances in cognitive decline-related disorders: from molecular mechanisms to clinical evidence
[J]. Oxid Med Cell Longev, 2019, 2019: 9695412. https://doi.org/10.1155/2019/9695412.Energy-stress-mediated AMPK activation inhibits ferroptosis
[J]. Nat Cell Biol, 2020, 22(2): 225-234. https://doi.org/10.1038/s41556-020-0461-8.Kaempferol ameliorates oxygen-glucose deprivation/reoxygenation-induced neuronal ferroptosis by activating Nrf2/SLC7A11/GPX4 axis
[J]. Biomolecules, 2021, 11(7): 923. https://doi.org/10.3390/biom11070923.Ferroptosis: death by lipid peroxidation
[J]. Trends Cell Biol, 2016, 26(3): 165-176. https://doi. org/10.1016/j.tcb.2015.10.014.Lithium chloride promotes neural functional recovery after local cerebral ischaemia injury in rats through Wnt signalling pathway activation
[J]. Folia Morphol, 2023, 82(3): 519-532. https://doi.org/10.5603/FM.a2022.0068.Brain-derived neurotrophic factor secreted by the cerebral endothelium: a new actor of brain function?
[J]. J Cereb Blood Flow Metab, 2018, 38(6): 935-949. https://doi.org/10.1177/0271678X18766772.Activation of α7 nicotinic acetylcholine receptors protects astrocytes against oxidative stress-induced apoptosis: implications for Parkinson’s disease
[J]. Neuropharmacology, 2015, 91: 87-96. https://doi.org/10. 1016/j.neuropharm.2014.11.028.NRF2, a transcription factor for stress response and beyond
[J]. Int J Mol Sci, 2020, 21(13): 4777. https://doi.org/10.3390/ijms21134777.Regulatory mechanisms of tetramethylpyrazine on central nervous system diseases: a review
[J]. Front Pharmacol, 2022, 13: 948600. https://doi.org/10. 3389/fphar.2022.948600.Structural basis of ligand binding modes of human EAAT2
[J]. Nat Commun, 2022, 13(1): 3329. https://doi.org/10.1038/s41467-022-31031-x.Folic acid ameliorates synaptic impairment following cerebral ischemia/reperfusion injury via inhibiting excessive activation of NMDA receptors
[J]. J Nutr Biochem, 2023, 112: 109209. https://doi.org/10.1016/j.jnutbio. 2022.109209.The role of AMPA and NMDA receptors in mitragynine effects on hippocampal synaptic plasticity
[J]. Behav Brain Res, 2023, 438: 114169. https://doi.org/10.1016/j.bbr.2022.114169.Astrocytes maintain glutamate homeostasis in the CNS by controlling the balance between glutamate uptake and release
[J]. Cells, 2019, 8(2): 184. https://doi.org/10.3390/cells8020184.Exposure to anandamide on young rats causes deficits in learning, temporal perception and induces changes in NMDA receptor expression
[J]. Behav Brain Res, 2023, 445: 114377. https://doi.org/10.1016/j.bbr.2023.114377.The dual roles of autophagy and the GPCRs-mediating autophagy signaling pathway after cerebral ischemic stroke
[J]. Mol Brain, 2022, 15(1): 14. https://doi. org/10.1186/s13041-022-00899-7.Autophagy inhibition exerts neuroprotection on white matter ischemic damage after chronic cerebral hypoperfusion in mice
[J]. Brain Res, 2019, 1721: 146337. https://doi.org/10.1016/j.brainres.2019.146337.Autophagy activation involved in hypoxic-ischemic brain injury induces cognitive and memory impairment in neonatal rats
[J]. J Neurochem, 2016, 139(5): 795-805. https://doi.org/10.1111/jnc.13851.Multisensory gamma stimulation mitigates the effects of demyelination induced by cuprizone in male mice
[J]. Nat Commun, 2024, 15(1): 6744. https://doi.org/10.1038/s41467-024-51003-7.Transcriptional convergence of oligodendrocyte lineage progenitors during development
[J]. Dev Cell, 2018, 46(4): 504-517.e7. https://doi.org/10.1016/j.devcel.2018.07.005.Mitophagy and quality control mechanisms in mitochondrial maintenance
[J]. Curr Biol, 2018, 28(4): R170-R185. https://doi.org/10.1016/j.cub.2018.01.004.RGD1564534 represses NLRP3 inflammasome activity in cerebral injury following ischemia-reperfusion by impairing miR-101a-3p-mediated Dusp1 inhibition
[J]. Exp Neurol, 2023, 359: 114266. https://doi.org/10. 1016/j.expneurol.2022.114266.Correction to: electroacupuncture ameliorates cognitive impairment through the inhibition of NLRP3 inflammasome activation by regulating melatonin-mediated mitophagy in stroke rats
[J]. Neurochem Res, 2022, 47(7): 1931-1933. https://doi.org/10.1007/s11064-022-03590-4.作者声称无任何利益冲突。
尹晴, 杨利. 脑梗死相关认知障碍相关机制[J]. 中南大学学报(医学版), 2024, 49(10): 1692-1699. DOI:10.11817/j.issn.1672-7347.2024.240213
YIN Qing, YANG Li. Pathophysiology of cognitive impairment related to cerebral infarction[J]. Journal of Central South University. Medical Science, 2024, 49(10): 1692-1699. DOI:10.11817/j.issn.1672-7347.2024.240213
http://xbyxb.csu.edu.cn/xbwk/fileup/PDF/2024101692.pdf
http://dx.chinadoi.cn/10.11817/j.issn.1672-7347.2024.240213

