1 Introduction
In 2022, the marine engineering construction industry in China achieved a total output value of 30 billion dollars, representing a 5.6% increase compared to the previous year. Several megaprojects, including cross-sea bridges, undersea tunnels, coastal ports, and offshore oil and gas engineering, were successfully undertaken, contributing to steady growth [1]. However, exposure to various factors in the marine environment, such as wave erosion, dry-wet freeze-thaw cycles, and the corrosive effects of Cl-, Mg2+, SO42- and CO32- ions in seawater, can lead to concrete structure damage and strength loss. Ensuring the durability of concrete in marine environments has become an urgent concern that needs to be addressed [2, 3].
Due to the presence of Cl- ions, seawater facilitates the formation of Friedel’s salt (3CaO⋅Al2O3⋅CaCl2⋅10H2O) and Kuzel’s salt (3CaO⋅Al2O3⋅0.5CaCl2⋅0.5CaSO4⋅10H2O) in the concrete through processes such as dissolution, precipitation, and ion exchange [4-6]. Among them, the expansive calcium oxychloride (CaCl2·3Ca(OH)2·12H2O) generates internal pressure within the concrete, leading to severe cracking. Mg2+ ions react with calcium hydroxide (CH) in concrete and form brucite, which reduces the alkalinity of the pore solution and ultimately compromises the stability of calcium-silicate-hydrate (C-S-H). Additionally, the presence of SO42- ions results in the formation of expansive ettringite (3CaO·Al2O3·3CaSO4·32H2O) and gypsum (CaSO4·2H2O), leading to the concrete expansion and cracking [7]. Therefore, the simultaneous presence of magnesium ions and sulfate ions in the chloride solution exacerbates the porosity and the volume of harmful pores. This in turn intensifies the infiltration of corrosive ions into the concrete matrix [8].
Ordinary Portland cement (OPC), known for its high strength and excellent durability, is currently the most widely used binder material in the field of concrete. It typically contains minerals such as C3S, C2S, C3A and C4AF, which react to form hydration products including C-S-H, CH and AFt [9]. However, the poor resistance to seawater corrosion makes it unsuitable for marine engineering applications. Calcium sulfoaluminate cement (CSA) consists primarily of C4A3S̅, C2S and C4AF, and its hydration products do not include CH, which is a raw material for harmful substances in seawater-corroded concrete [10]. Ferrite-rich calcium sulfoaluminate (FCSA) cement and CSA belong to the same type of cement, differing mainly in the iron phase. When the content of CaO is elevated in cement raw materials, it serves to impede the formation of gehlenite, leading to a reduction in the iron content within ye’elimite. Consequently, this results in an elevated ferrite content [11]. High-iron ferrite minerals possess a high Fe/Al ratio, which transforms AFt from a needle-like morphology to a uniformly elongated rod-shaped structure. This transformation leads to the formation of a dense covering layer that controls the growth of AFt, effectively impeding ion dissolution, the hexacalcium aluminodiferrite (C6AF2) demonstrates improved resistance to sulfate attack [12, 13]. Moreover, when the iron replaces aluminum in calcium aluminate, the resulting crystal structure becomes more stable compared to iron-free calcium aluminate [14]. FCSA cement contains Al-rich ferrite, specifically C6AF2, which imparts superior resistance to seawater corrosion due to its higher iron content. The primary hydration products of FCSA cement are AFt, iron hydroxide gel (FH3), and aluminum hydroxide gel (AH3). A significant amount of AFt forms during the early hydration of FCSA cement, contributing to a dense structure, while AH3 and FH3 gels further fill in the pores and refine the pore structure. This makes FCSA cement promising for marine engineering applications [15, 16].
Mineral admixtures, as by-products of industrial production, are widely used in the field of building materials [17]. During the early hydration of FCSA cement, there is a significant and concentrated heat release [16]. The addition of mineral admixtures can reduce the early heat release and prevent cracking due to temperature stress, although it may have a negative impact on compressive strength [18]. In seawater environments, the pore size of concrete tends to increase over time. The proper incorporation of mineral admixtures can enhance the structural densification of cementitious slurries [19], refine the micro-pore structure of cement-based materials [20], improving the pore characteristics of concrete, and enhancing its durability [21, 22]. Particularly in corrosive environments containing SO42- and Mg2+, the addition of slag significantly improves the compressive strength, resistance to Cl- intrusion, and stability of hydration products [9]. Previous studies have shown that finely ground granulated blast furnace slag exhibits excellent performance in enhancing the durability of concrete in marine environments [23, 24]. Furthermore, mineral admixtures can improve the resistance to chloride ion permeability and maintain mechanical properties after the seawater corrosion [2, 7].
Therefore, this study aims to investigate the performance evolution of FCSA cement paste in a seawater environment. Additionally, GGBS primarily exerts filling and dilution effects on the mechanical properties of FCSA, having both positive and negative impacts on the properties of specimens. Given the economic and environmental benefits of substituting GGBS, this study also aims to explore the effects of partially replacing the cement paste with GGBS to understand the impact of this mineral admixture on FCSA cement paste.
2 Experimental procedures
2.1 Materials and sample preparation
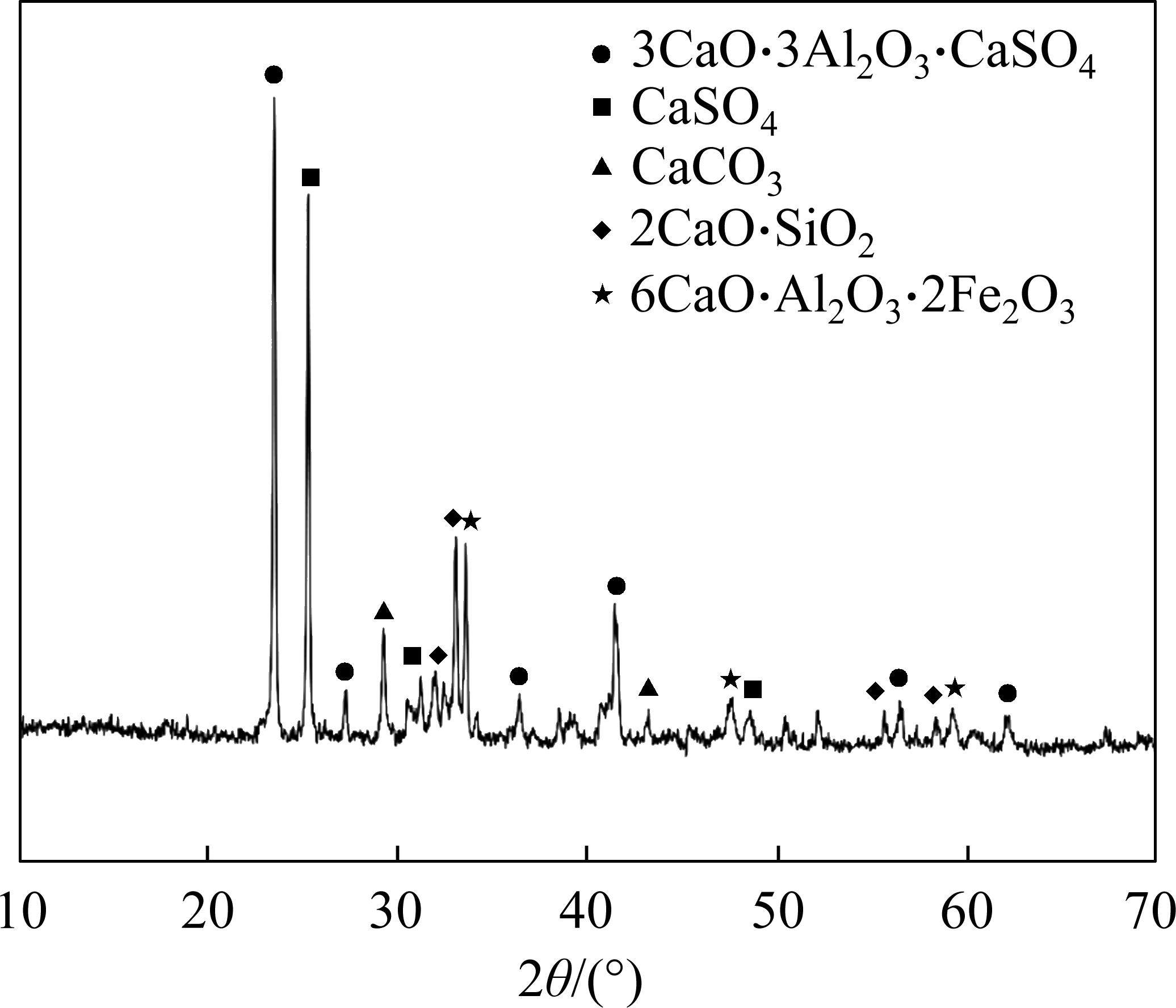
The research employs commercial FCSA cement. The X-ray diffraction (XRD) pattern is depicted in Figure 1, while the chemical composition of utilized raw materials is detailed in Table 1. The primary mineral constituents encompass ye’elimite (C4A3S̅), anhydrite (CS̅), belite (β-C2S), hexacalcium aluminodiferrite (C6AF2), along with trace amounts of calcite (CaCO3). Table 2 presents the composition breakdown of the major minerals within FCSA cement.
| Composition | CaO | Al2O3 | SO3 | SiO2 | Fe2O3 | MgO | TiO2 | Na2O | K2O | LOI |
|---|---|---|---|---|---|---|---|---|---|---|
| FCSA | 41.91 | 22.42 | 11.21 | 7.16 | 6.43 | 0.12 | 1.18 | — | 0.12 | 3.75 |
| GGBS | 44.02 | 12.38 | 2.07 | 29.25 | 0.92 | 7.79 | 1.34 | 0.55 | 0.47 | 1.21 |
 |  |  |  | 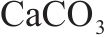 |
|---|---|---|---|---|
| 39.7 | 25.6 | 18.6 | 11.5 | 4.6 |
The standard consistency water requirement for FCSA cement is measured at 25.7% by mass of the cement. The initial setting time and final setting time are 27 and 31 min, respectively. Compressive strength of the cement is measured at 32.8, 43.4, and 50.2 MPa at 1, 3 and 28 d, respectively. Finely GGBS serves as the mineral admixture. Figure 2 illustrates the XRD pattern, showcasing anhydrite, quartz, gehlenite, and calcite as primary constituents.
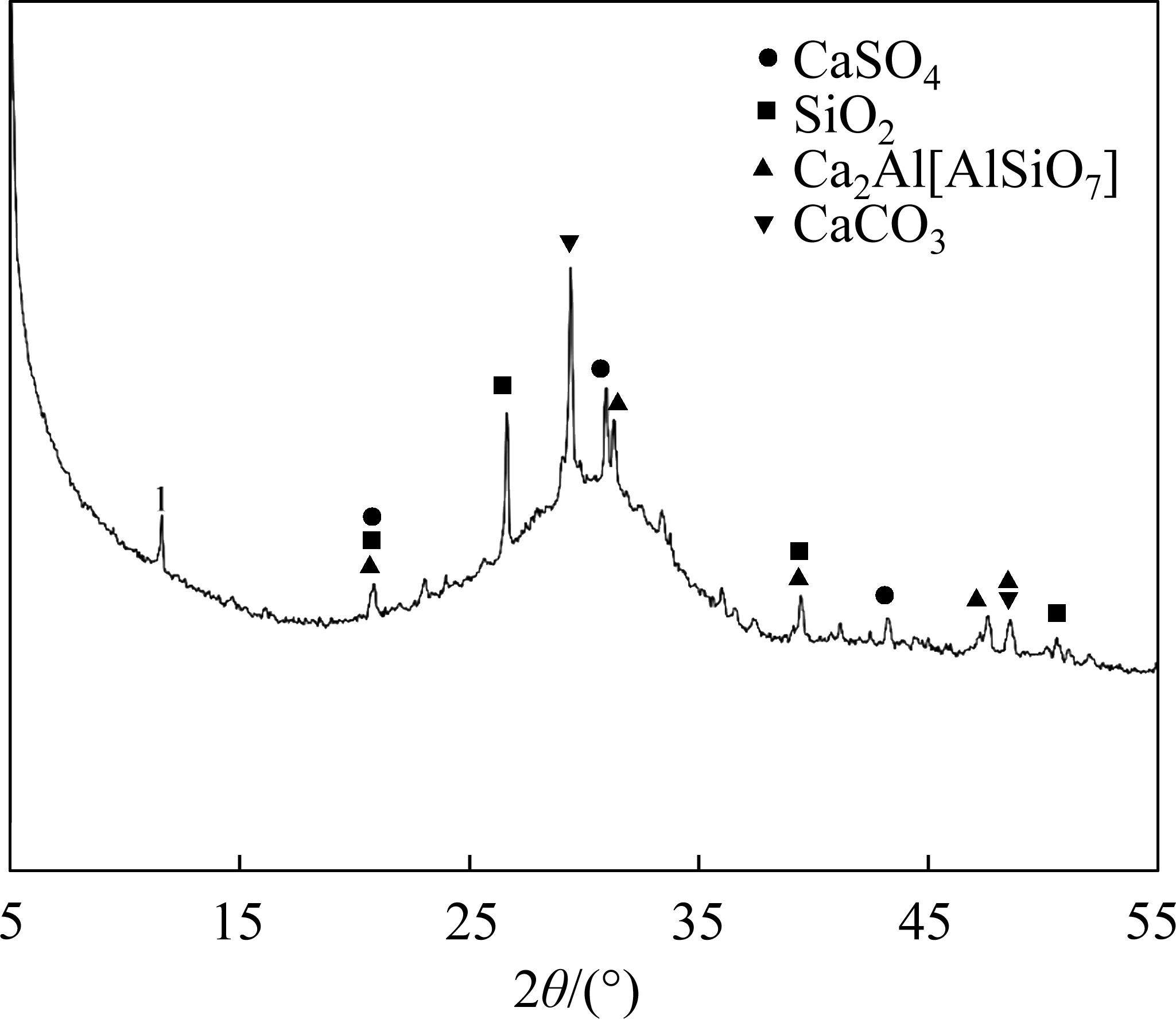
The particle size distribution of raw materials is presented in Figure 3. Median particle sizes for FCSA cement and GGBS are 21.55 and 14.47 µm, respectively. Their particle size distributions span ranges are 0.36-208.93 µm and 0.96-69.18 µm, respectively.
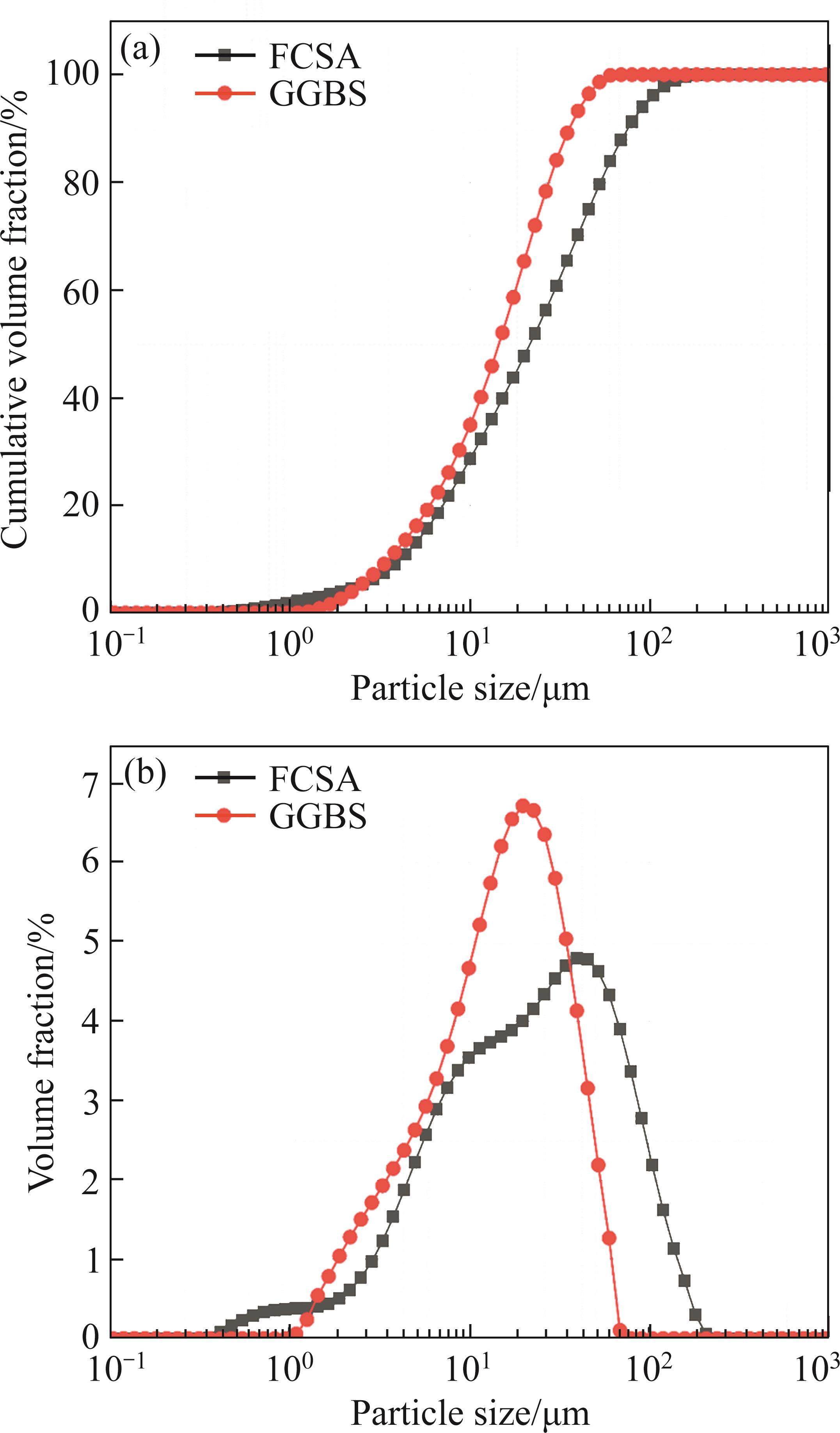
Comparatively, GGBS exhibits a narrower particle size distribution than FCSA cement, with increasing GGBS content leading to decreased cement paste flowability. The inclusion of GGBS has marginal influence on the setting time of FCSA cement.
In this study, the contents of GGBS were set as 0%, 10%, 20% and 30%, respectively, as an equivalent replacement of FCSA cement by weight. The cement paste test specimens were prepared in accordance with the standards specified in GB/T 17671—2021. The test pieces have dimensions of 40 mm×40 mm×40 mm. A water-to-cement ratio of 0.45 was used, with tap water serving as the mixing water. Mix proportions and workability of cement paste were showed in Table 3. Fluidity and setting time assessments adhered to GB/T 1346—2011 and GB/T 8077—2012, respectively. The curing solution was tap water and seawater. The seawater with a pH value of 8.2 was prepared according to ASTM D1141-98(2013). The chemical composition of the seawater is shown in Table 4. To ensure the stability of the ion concentration, seawater was replaced once a month. After being cured for 24 h under standard curing conditions at a temperature of approximately 20 ℃ and relative humidity of 95% or higher, all specimens were demolded. Subsequently, they were further cured in tap water for 48 h to improve their early corrosion resistance. Finally, the specimens were transferred to tap water and seawater for curing until the desired test age was reached. It should be noted that certain test parameters in the seawater were measured after a curing period of 3 d in the tap water.
| Code | Mass fraction/% | w/b | Fluidity/mm | Setting time/min | ||
|---|---|---|---|---|---|---|
| FCSA | GGBS | Initial | Final | |||
| Control | 100 | — | 0.45 | 174 | 95 | 112 |
| SL10 | 90 | 10 | 0.45 | 155 | 96 | 108 |
| SL20 | 80 | 20 | 0.45 | 150 | 98 | 109 |
| SL30 | 70 | 30 | 0.45 | 147 | 100 | 111 |
| NaCl | MgCl2 | Na2SO4 | CaCl2 | KCl | NaHCO3 |
|---|---|---|---|---|---|
| 24.53 | 5.20 | 4.09 | 1.16 | 0.695 | 0.201 |
2.2 Test methods
An isothermal calorimeter (TAM Air) was utilized to characterize the early heat release during the hydration of FCSA cement paste, as well as to examine the influence of mineral admixtures on the heat release. The water-binder ratio employed was 0.45, and the temperature during testing was maintained at (20±2) ℃ for a duration of 72 h.
X-ray diffraction (XRD) analysis was employed to analyze the evolution of hydration products. The cured samples were ground into powder at the desired age and subsequently immersed in absolute ethanol to halt the hydration process. Prior to conducting the micro test, the samples were retrieved, dried, and passed through a 45 µm sieve. Approximately 1-2 g of each sample was taken for analysis. The scanning range for XRD analysis was set at 5°-90° with a scanning speed of 5°/min.
Thermogravimetric analysis (TG/DTG) is a supplementary technique for the analysis of gel-like substances in hydrated products, and the test samples were taken from the same batch used for XRD analysis. In the test, the powdered sample was heated from 30 ℃ to 1000 ℃ at a rate of 10 ℃/min under the protective atmosphere of N2. Each sample weighed about 1 g.
The compressive strength of the specimen was tested according to the Chinese standard GB/T 17671—2021, and the load growth rate was (2.4±0.2) kN/s. The coefficient of corrosion (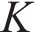
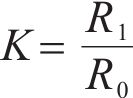
where R1 and R0 represented the compressive strength of the test specimen in seawater and at the same age in tap water at 20 ℃, respectively.
The 40 mm×40 mm×40 mm specimen was immersed in seawater, and the mass was measured at different time intervals to observe any changes. Before each measurement, the specimen was thoroughly washed three times with deionized water to remove any salt residue floating on its surface. Subsequently, it was gently dried with a dry cloth and then weighed to determine the mass.
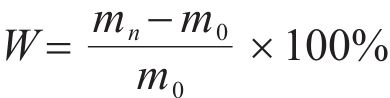
where W was the mass change rate; m0 and mn were the mass before and after immersion in seawater.
The pore solution of the cement paste was extracted using the solid-liquid extraction method [25]. After removing the sample from the curing solution, the surface impurities were washed off with deionized water and then dried. The dried sample was ground into a fine powder. Approximately 5 g of the ground sample was weighed and mixed with 50 mL of deionized water. The mixture was stirred using a magnetic stirrer for 30 min and then allowed to settle for 2 h. The pH value and conductivity of the supernatant were subsequently measured.
Before conducting the MIP test, the cured samples at the desired age were crushed, and approximately 1 cm3 cubic samples were taken from the middle of the samples. These samples were then immersed in ethanol to stop the hydration of cement pastes by removing free water. Before testing, the samples were dried to a constant weight in a vacuum drying oven. The selected samples for testing were cured in seawater for a period of 90 d.
3 Results and discussion
3.1 Heat of hydration
The hydration heat release curves of FCSA cement are shown in Figure 4. FCSA cement paste exhibits a relatively fast heat release rate, with a significant portion of the heat being released within 24 h of hydration. GGBS exhibits lower hydration heat, reducing the overall heat release of the cement paste [26]. From Figure 4, it can be observed that FCSA cement exhibits four distinct exothermic peaks. The initial declining segment corresponds to the exothermic peak associated with cement particle dissolution. However, a complete peak is not clearly observed in Figure 4.
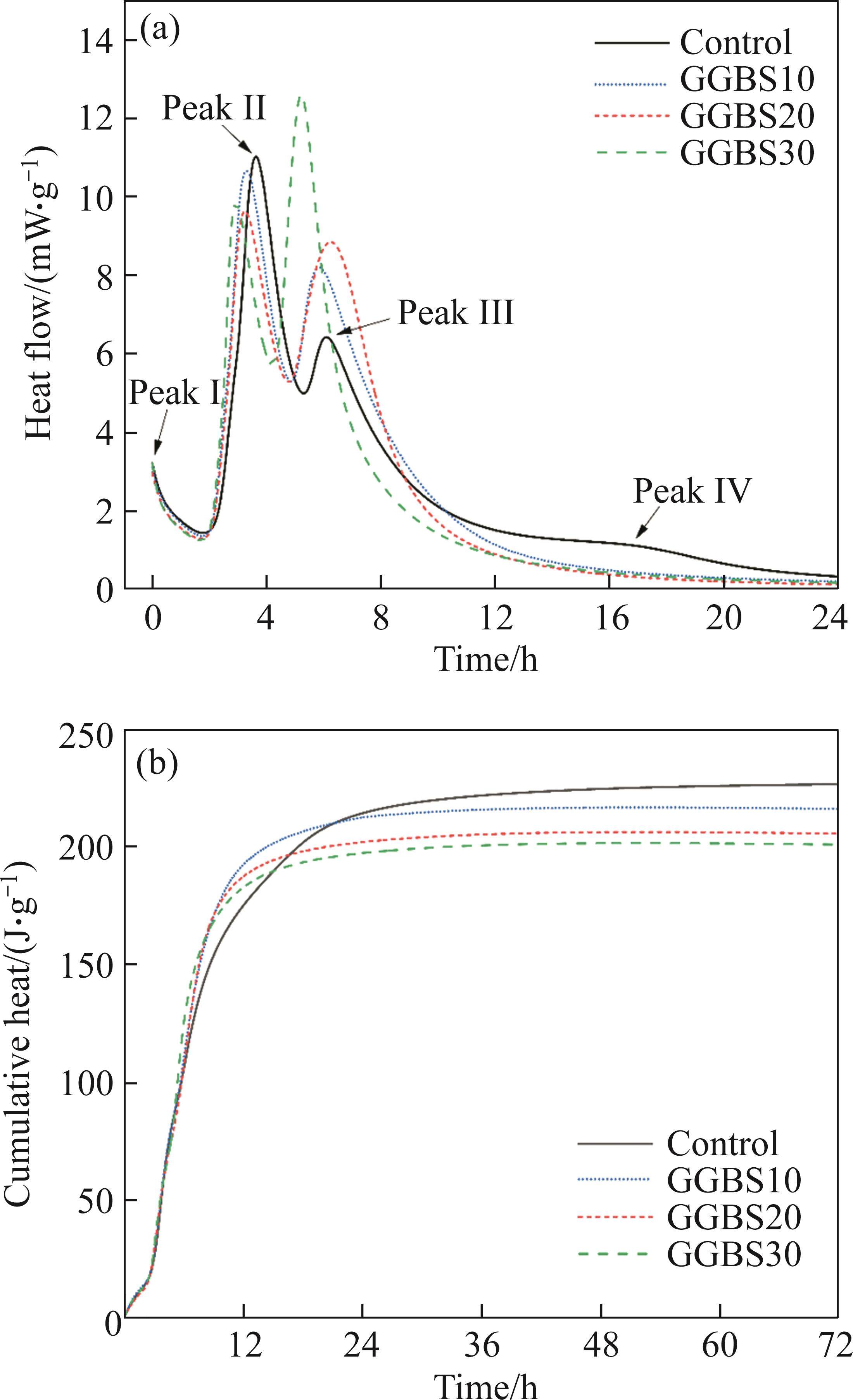
The second exothermic peak of FCSA cement occurs at approximately 4 h and is primarily attributed to the hydration reaction of ye’elimite with gypsum, as indicated in Eq. (3). This reaction forms the main hydration product, AFt, releasing a significant amount of heat. The addition of GGBS results in a decrease in the second peak value of the exothermic peaks from 11.07 mW/g to 10.65 mW/g, 9.65 mW/g, and 9.82 mW/g, respectively. This reduction can be attributed to the partial substitution of cement in the slurry, which leads to a decrease in the formation of AFt.
After the abundant formation of AFt, it precipitates as a thin film on the surface of cement particles, hindering further hydration and slowing down the exothermic reaction. Subsequently, under the influence of osmotic pressure, this layer of AFt film ruptures, allowing cement particles to continue dissolving and hydrating, leading to the appearance of a third exothermic peak [27]. The third exothermic peak occurs at around 6 h and may be attributed to the localized formation of AFt or monosulfoaluminate (AFm) within the hydration product layer surrounding cement particles, possibly under the control of ion diffusion. The exothermic peaks of different samples are 6.44, 8.19, 8.86 and 12.63 mW/g, respectively. The inclusion of GGBS significantly increases the value of the third exothermic peak by 2.42 mW/g compared to the control sample. The aluminum phases in the GGBS gradually dissolve and react with gypsum under early alkaline conditions, resulting in the formation of AFt. The gypsum consumption creates conditions for AFm generation in this process, and the higher the GGBS content, the more it enhances the hydration heat release at this stage. Moreover, the additional Ca2+ from the GGBS entering the solution promotes the formation of AFt [28]. Moreover, GGBS has finer particles and a larger specific surface area compared to FCSA cement, providing more nucleation sites for hydration, which accelerates the hydration reaction. It is worth noting that although high hydration heat usually represents a high degree of hydration and many hydration products, the compressive strength of the cement paste is not only dependent on the hydration products and degree of hydration but is also significantly influenced by the volume of pores within the paste matrix [29].
The fourth exothermic peak is not clearly pronounced and appears approximately at 16 h, which could be associated with the hydration of iron phases. And the sample with GGBS shows the minimal or negligible presence of this peak. Overall, GGBS exhibits low heat of hydration, and its dilution effect reduces the overall heat release of the cement paste.
3.2 Hydration products
Figure 5 presents the XRD patterns of FCSA cement paste after being cured in tap water for 3 d, 28 d, and 90 d. Additionally, Figure 6 displays the XRD patterns of FCSA cement paste cured in seawater for 28 d and 90 d. Notably, all samples underwent an initial 3-day curing period in tap water before being immersed in seawater. As a result, the seawater-cured samples lack XRD patterns for the 3-day curing duration. For the tap water-cured samples, the main hydration products of FCSA cement include AFt, AFm, gypsum, calcite, and unhydrated belite. As the hydration time extends from 3 d to 28 d, the diffraction intensity of AFt shows little change, indicating a rapid hydration rate of FCSA cement paste. The formation of a significant amount of AFt at 3 d supports early strength development. In the presence of an ample gypsum content, the hydration of FCSA cement gives rise to a significant formation of AFt. Conversely, in cases where there is an insufficient supply of gypsum, the hydration of ye’elimite leads to the formation of AFm [30]. Gypsum can be still detected in the samples cured for 90 d, indicating the limited formation of AFm. Compared to the control specimen, GGBS20 showed no significant differences in the types of hydration products. However, the overall peak intensities were lower than those of the Control sample, primarily due to the partial replacement of cement by GGBS, which specifically resulted in a reduced formation of hydration products such as AFt.
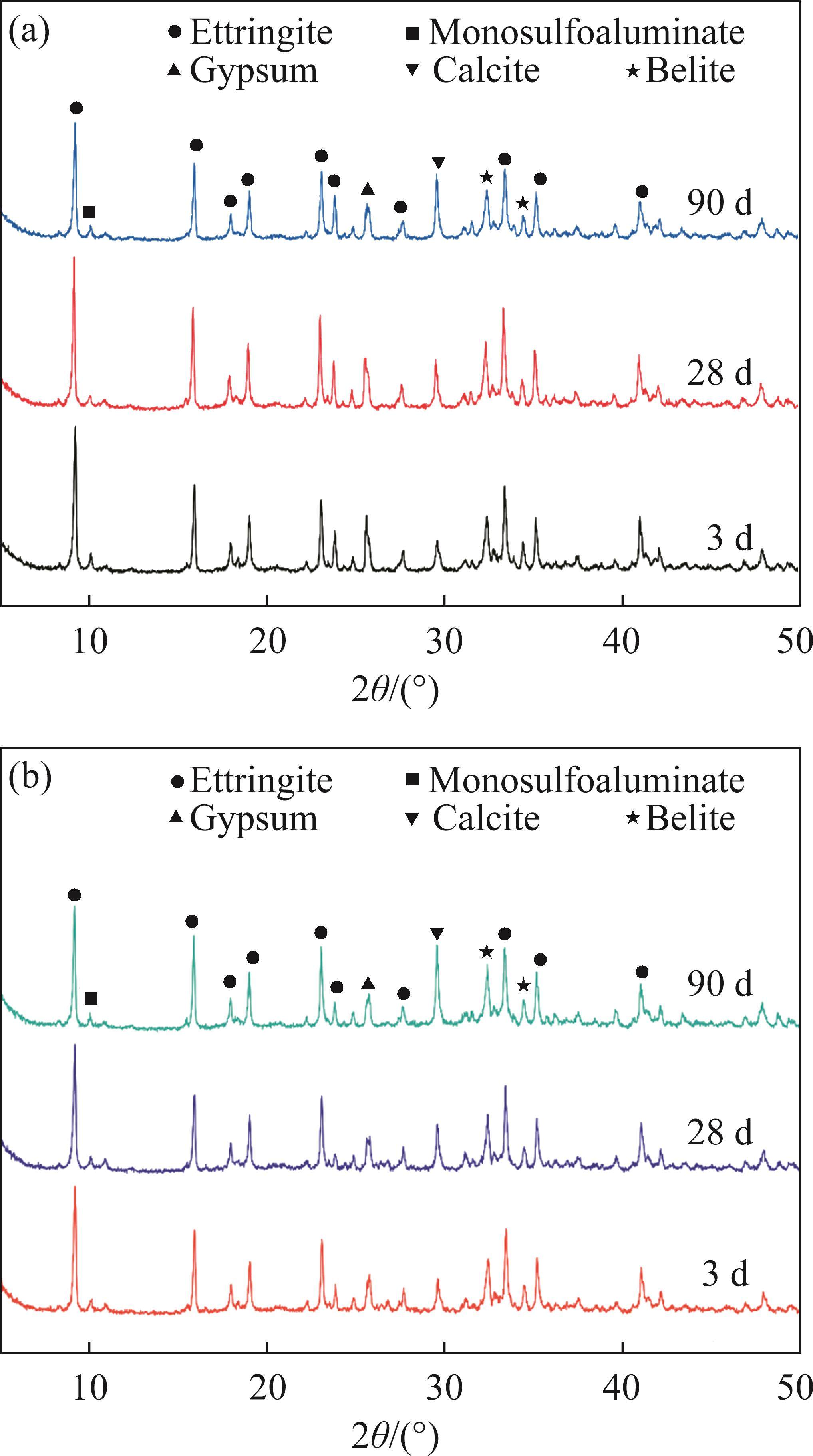
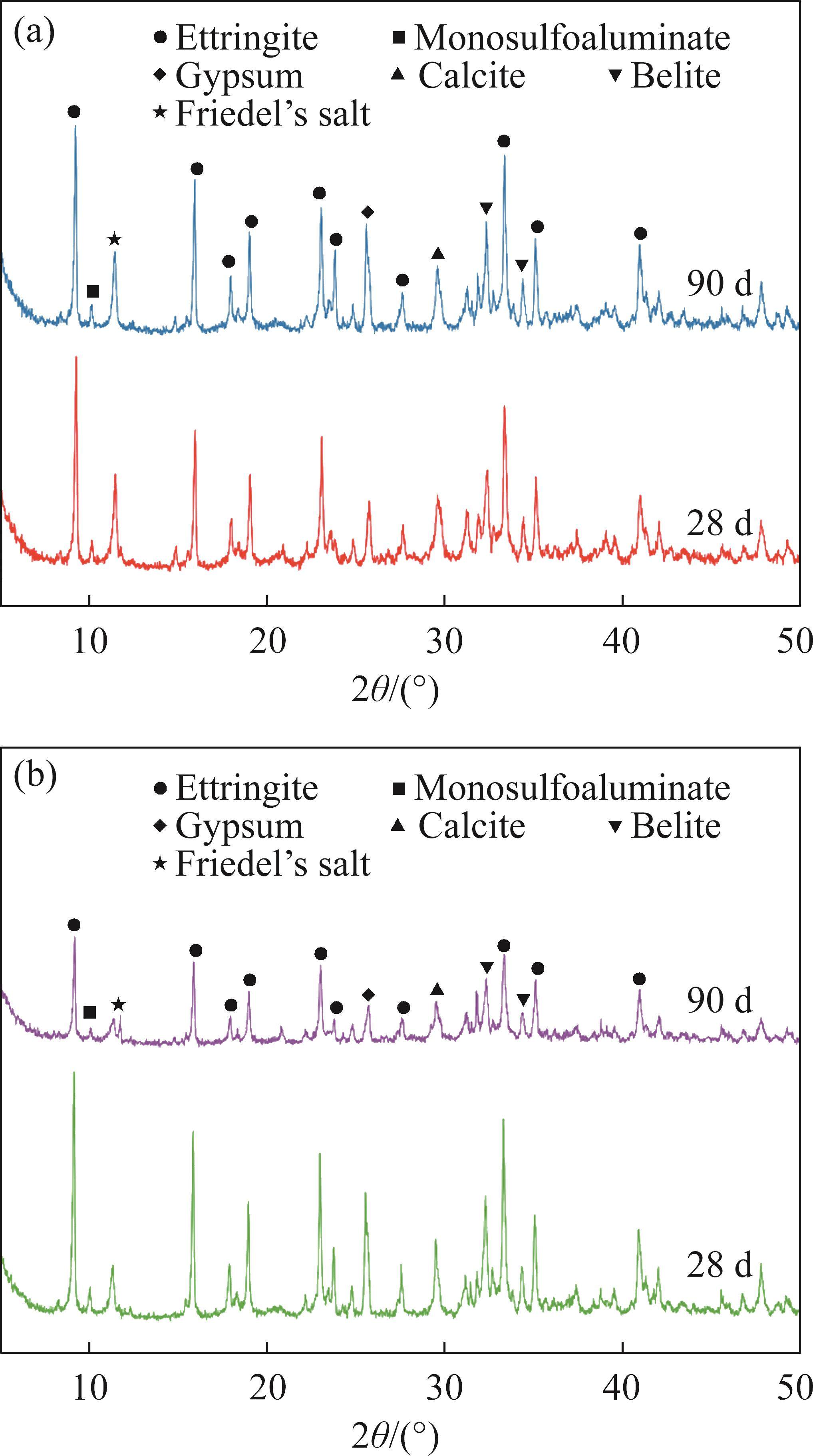
As the samples solidify in seawater, there is an increase in the gypsum content. This is attributed to the high concentration of Cl- in the environment, which disrupts the structure of some AFt and AFm phases, leading to their combination and the formation of Friedel’s salt and gypsum. AFm possesses a layered structure, where the positively charged main layer consists of [Ca2Al(OH)6]+ units. The positive charge of this layer is balanced by X- anions (such as SO42-, Cl-, CO32-) presented in the interlayer, making AFm highly sensitive to the concentration of anions in the pore solution [6]. After a certain period of curing in the seawater, Cl- that penetrates into the pores partially replaces the SO42- in AFm, resulting in the formation of Friedel’s salt [31], as described in Eq. (6). The amount of Friedel’s salt generated is dependent on the value of C4A3S̅/CS̅, where a high value indicates a great amount of AFm produced and a strong ability to capture chloride ions through chemical reactions, leading to the formation of more Friedel’s salt [32]. Friedel’s salt also contributes to the further filling of the sample’s pores, resulting in a dense structure. When the curing time is extended from 28 d to 90 d, there is a reduction in the intensity of AFt diffraction peak, indicating that the prolonged exposure of samples to seawater leads to partial hydrolysis of AFt. For the semi-quantitative analysis of the XRD test results, at 90 days of curing, the AFt content in the Control and GGBS20 samples under tap water curing conditions was 53.26% and 49.21%, respectively. Compared to the values under seawater curing, which were 45.32% and 39.96%, these amounts decreased by 7.94% and 9.25%, respectively. As the hydration time extended beyond 28 d, the absence of CH detected in the hydration products of FCSA cement indicated that no excess CH was available to react with sulfate ions, leading to minimal additional AFt formation. Furthermore, the existing AFt in the original specimens gradually decomposed over time, resulting in a reduction in AFt content to some extent at 90 d. Additionally, under the sulfate attack, CH can cause expansion and deterioration of concrete. However, since CH was not detected in the XRD analysis of this study, this is one of the important factors contributing to the seawater corrosion resistance of FCSA cement paste.
XRD primarily characterizes crystalline materials, while TG-DTG analysis is needed to complement gel-type hydration products. Figures 7 and 8 depict the TG-DTG curves of FCSA cement paste at different curing times in tap water and seawater, respectively. The curves exhibit approximately four distinct mass loss peaks, corresponding to different hydration products.
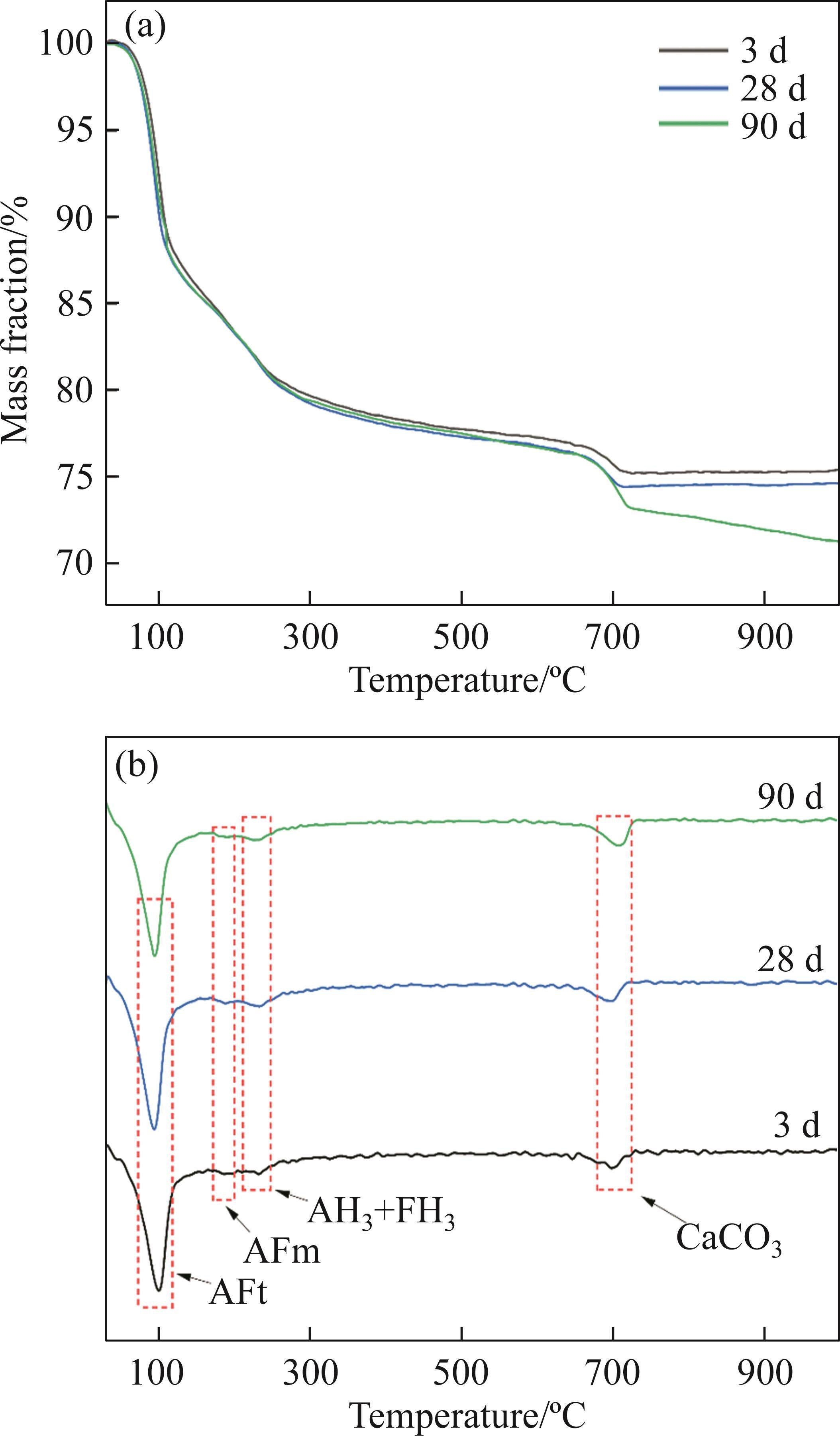
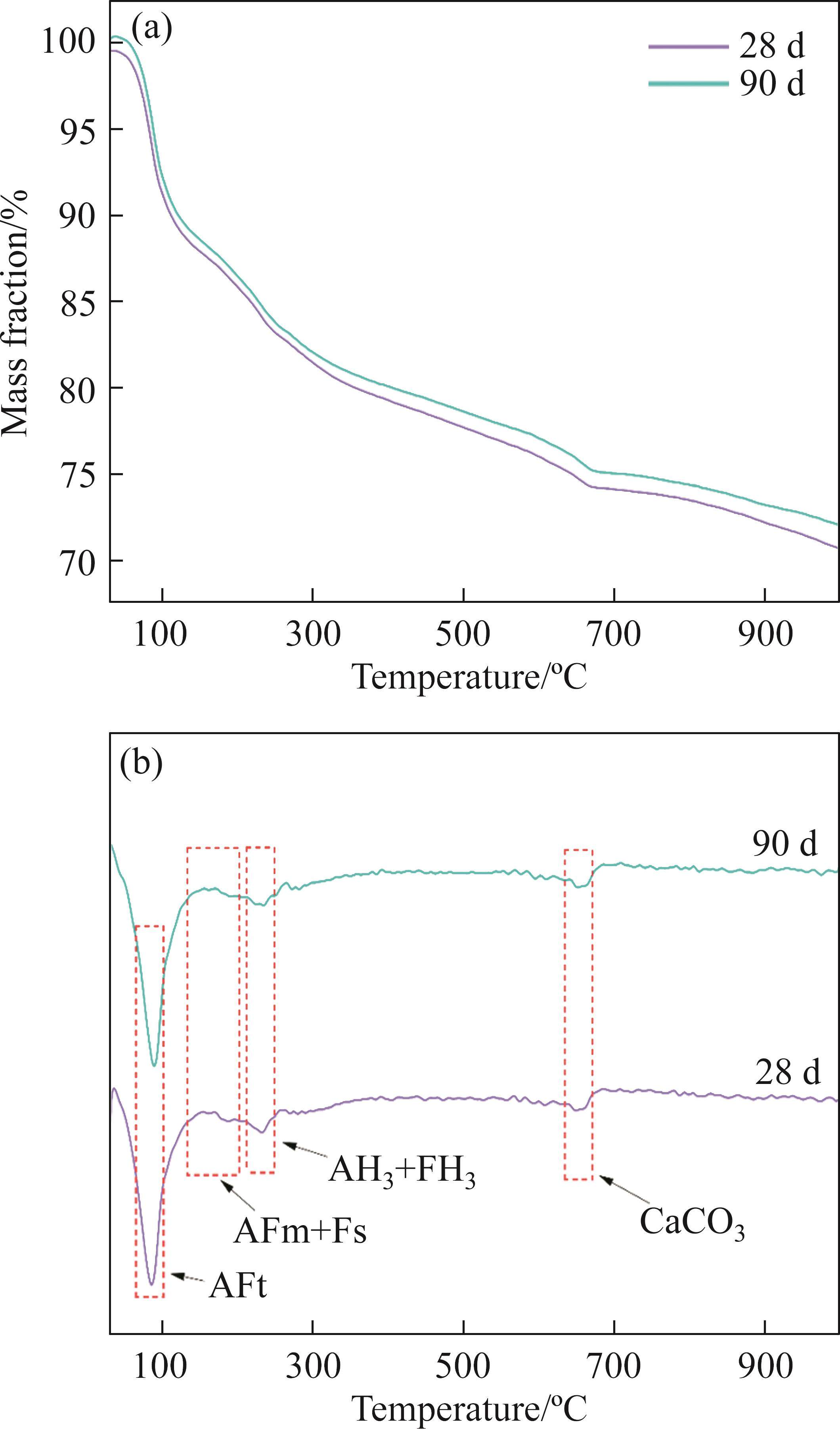
For samples cured in tap water, the first peak, observed at 50-120 ℃, is attributed to the dehydration of AFt. After 3 d of hydration, a substantial amount of AFt has formed, and the quantity increases with the progression of hydration time until 28 d. However, at 90 d, there is a slight decrease in AFt, possibly indicating an insufficient gypsum content during later stages of hydration, leading to the partial transformation of AFt into AFm. Around 180 ℃, a peak is caused by the dehydration of AFm, but its generation is relatively limited and not prominently visible in Figure 7. The dehydration peaks of AH3 and FH3 occur at approximately 230 ℃. The final peak observed between 650 and 720 ℃, results from the decomposition of CaCO3, and its formation increases with curing time. On one hand, CaCO3 is introduced from the original FCSA cement paste, and on the other hand, it may be related to the hydration of C2S. C2S exhibits a slow early hydration rate, and during the later stages of hydration, CH is formed, and there is an increase in the production of CaCO3 following carbonation.
For samples cured in the seawater, an additional dehydration peak of Friedel’s salt is observed between 130 and 200 ℃. Furthermore, compared to the 28-day cured samples in the seawater, the mass loss rate of 90-day cured samples is slightly lower. This observation suggests that under seawater exposure, some of the hydrated products should undergo hydrolysis.
3.3 Compressive strength
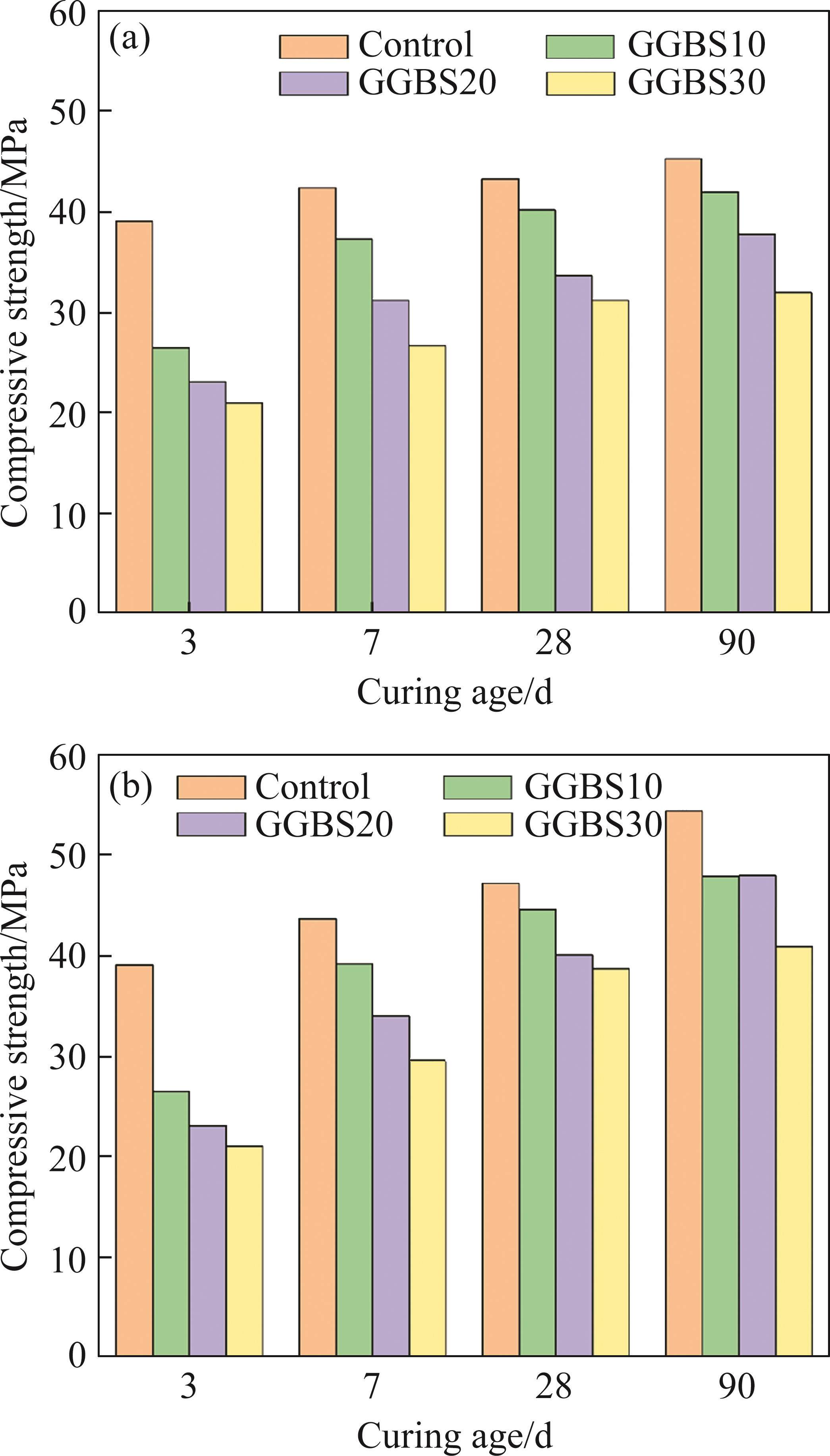
Figure 9 illustrates the compressive strength of different specimens, encompassing two diverse curing environments: tap water and seawater. Notably, a preliminary tap water curing period of three days precedes the immersion of specimens in seawater. In two different curing environments, the compressive strength of FCSA cement gradually increases with age. FCSA cement exhibits a relatively rapid rate of hydration, leading to the early formation of hydration products. The later stages of hydration also maintain a steady growth in strength. AFt contributes to the initial strength development of FCSA cement pastes, while unreacted C6AF2 and C2S phases may potentially augment the strength during the later stages of hydration [33]. When cured in seawater, the specimens experience a faster increase in compressive strength. The presence of SO42- in seawater to some extent promotes the formation of AFt, and the formation of Friedel’s salt contributes to a more compact cementitious structure. After adding GGBS, the strength of the specimens continued to increase during the 90-day curing period. GGBS can contribute to the strength of cement paste through the pozzolanic effect of volcanic ash. In the later stages of hydration, CH released from C2S hydration promotes the hydration of GGBS to some extent. However, the limited generation of CH in FCSA cement pastes results in the activity of GGBS not been fully exerted, leading to a decline in the strength of the cement paste. Additionally, MATTHEW et al [34] studied the corrosion resistance mechanism of GGBS in seawater by increasing alkalinity. The results indicate that the strength brought about by the reaction between GGBS and the matrix increases over time. Therefore, using GGBS as a mineral admixture to replace cement for resisting seawater corrosion is feasible, despite the reduction in strength due to the partial replacement of cement by GGBS.
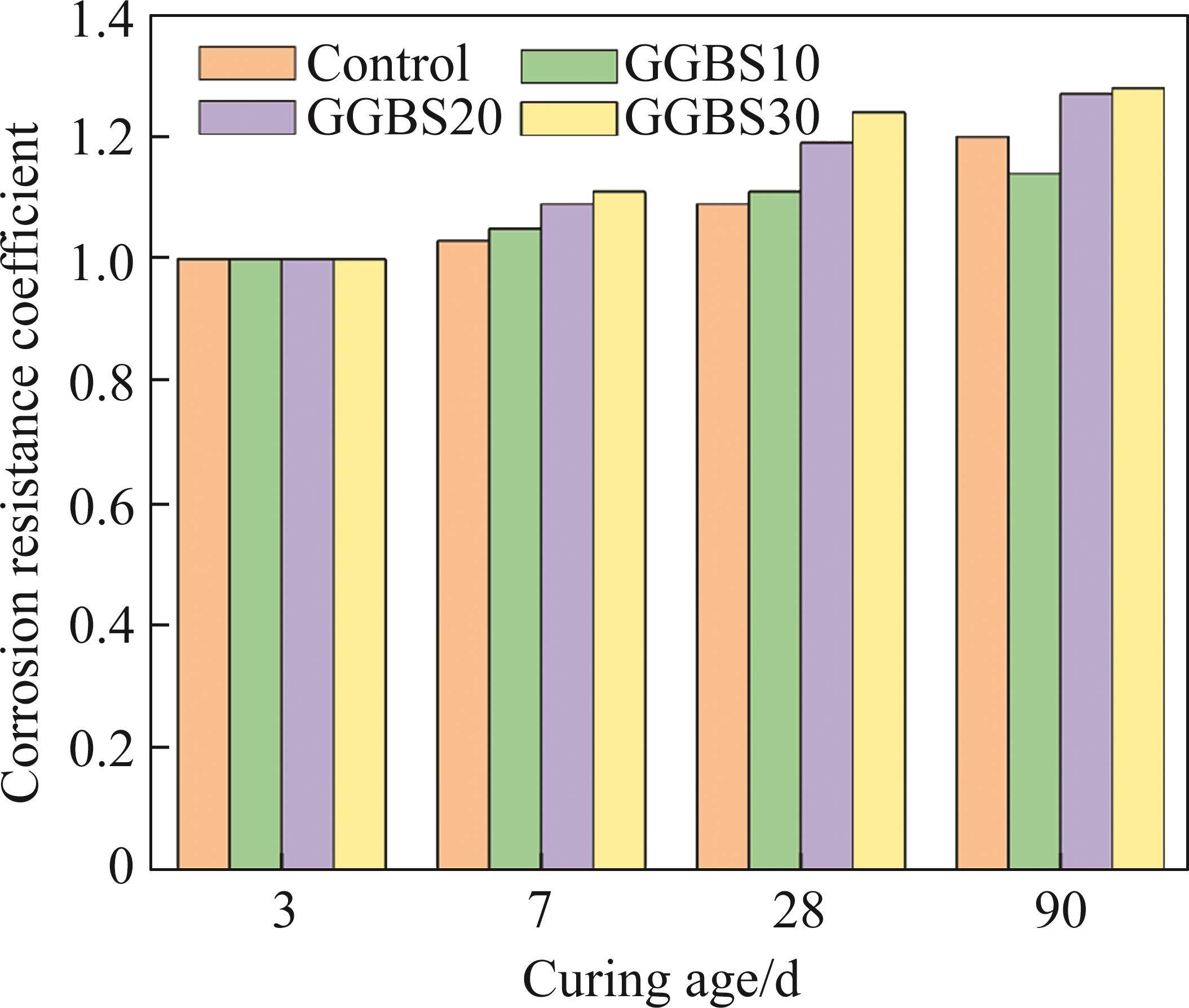
3.4 Coefficient of corrosion
Figure 10 illustrates the coefficient of corrosion of the specimens cured in seawater for different durations. All coefficients of corrosions are larger than 1 and exhibit a gradual increase with extended curing time. Compared to tap water curing, curing in seawater results in the additional formation of Friedel’s salt, which enhances the compactness of the cementitious structure and improves the mechanical performance of the system [35]. The addition of mineral admixtures to a certain extent improves the coefficient of corrosion of FCSA cement, possibly due to the filling effect of mineral admixtures, which increases the density of the cementitious structure and slows down the formation of expansion products such as calcium aluminate and gypsum, reducing the risk of microcracks caused by expansion stress [36]. The addition of GGBS refines the pore structure and reduces the porosity of the specimens. Besides, compared to the low reactivity of GGBS during curing in tap water, the presence of SO42- in seawater promotes the hydration of the aluminate phases in GGBS, leading to the formation of many AFt. With high GGBS content, the difference in compressive strength between specimens cured in tap water and seawater increases. This is also the reason for the high coefficient of corrosion observed as the GGBS content increases.
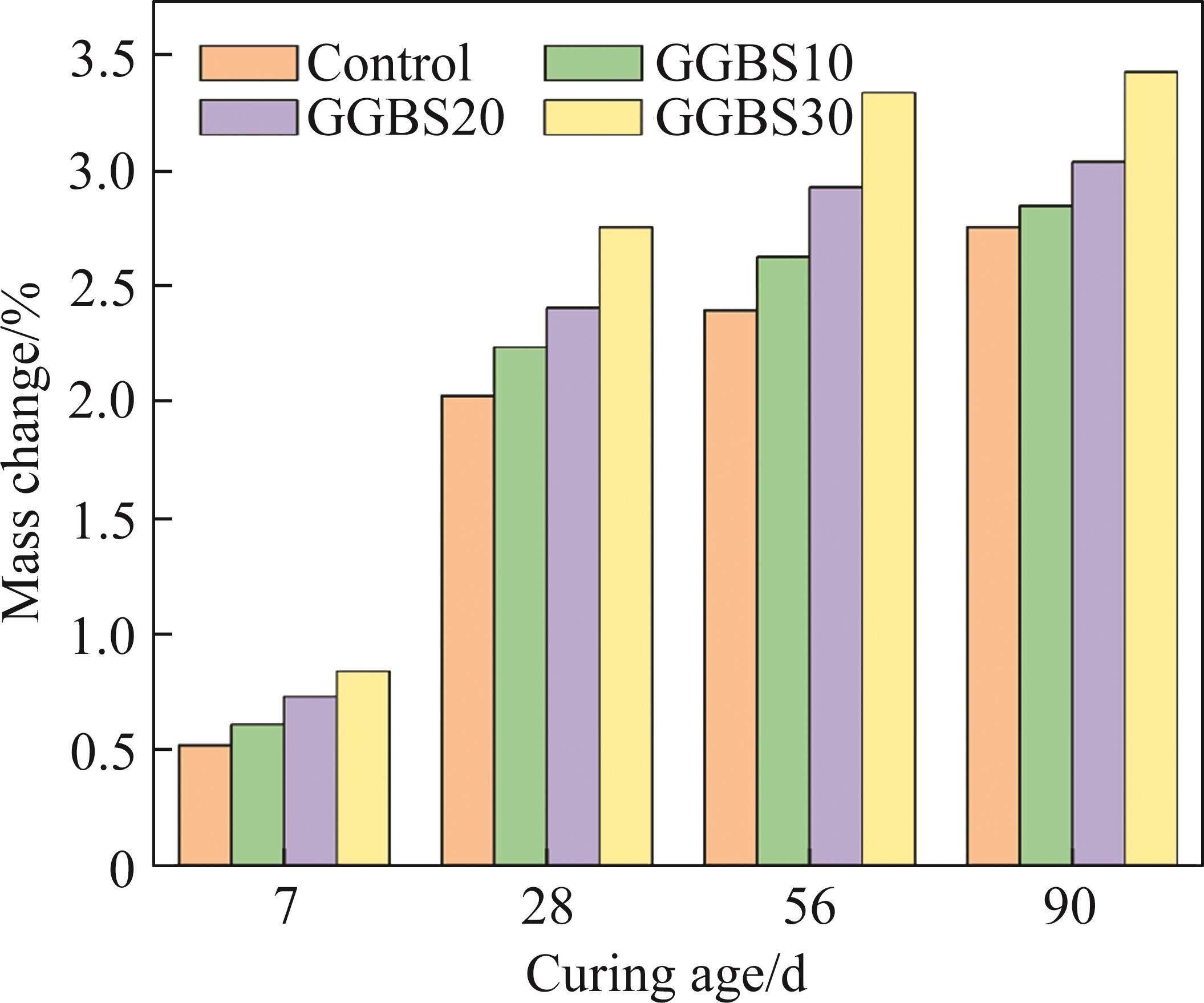
3.5 Mass change
When seawater infiltrates into the interior of the cementitious material through its porous structure due to the external erosion, the contact between the external seawater and the pore solution inside the specimen alters the static water equilibrium, resulting in a net mass flow. The seawater infiltration and ion transport caused by fluid movement can be classified into the saturated flow and unsaturated flow [37]. Under unsaturated conditions, the transport mechanism of the cementitious material primarily relies on the absorption of pore structure capillary suction and the permeation driven by hydraulic gradients [38]; under saturated conditions, the diffusion driven by chemical potential differences transports solutes from high concentration areas to low ones, and chemical reactions also affect ion transport [2].
Figure 11 illustrates the mass changes in different systems cured in seawater, and the increase in specimen mass can be mainly attributed to: 1) the evolution of hydration processes and the formation of hydration products [39]; the presence of sulfates in seawater promotes the generation of AFt [40]; 2) the accumulation of salts on the surface and within the pores of the specimen; 3) the water absorption rate of the internal system of the specimen. Overall, the mass of the specimens gradually increases with age. In the early stages, the pore solution is in an unsaturated state, and a large amount of seawater infiltrates into the specimens. The gel-like substances formed by FCSA cement provide a large surface area, allowing the seawater to adhere to the specimen surface [41], resulting in rapid mass growth. As the exposure time increases, the internal pores of the specimen become saturated with seawater, leading to slow mass growth in the later stages [42]. With increasing mineral admixture content, the mass growth of the specimens becomes significant. Although the mineral admixture partially replaces cement and plays a partial filling role, it fails to generate dense hydration products, resulting in a high accumulation of salts within the specimens.
3.6 Pore solution
Figure 12 shows the pH values of the pore solution for different samples. In tap water, with the prolongation of curing time, the pH values of the samples exhibit an initial increase followed by a decrease. In the early stages, cement particles rapidly dissolve and hydrate, releasing a substantial number of alkaline ions. This process leads to a rapid increase of pH value in the pore solution. Simultaneously, gypsum is continuously consumed, causing the concentrations of sulfate and calcium ions to decrease. In order to maintain the electrical neutrality of the pore solution, there is an increase in the concentration of hydroxide ions, consequently resulting in a continuous rise in the pH value [43]. Furthermore, the fluid within the pores continues to be consumed due to the ongoing formation of hydration products, further amplifying the alkaline concentration. The pH value of the samples after 28 d of hydration is lower than that at 7 d, the trend is attributed to the sustained consumption of alkaline ions and the generation of hydration products. Upon partially replacing cement with GGBS, an increase in the pH value of the pore solution is noted during the initial stages. The increase in pore solution alkalinity due to GGBS may be related to the release of alkaline ions. The relatively low alkalinity of the pore solution in FCSA cement promotes the extraction of alkaline ions from the mineral admixture, leading to ion exchange in the pore solution and a slight increase in its alkalinity [44]. However, the gradual hydration of GGBS during the subsequent stages leads to the consumption of OH- ions within the pore solution, subsequently resulting in a reduction of the pH value.
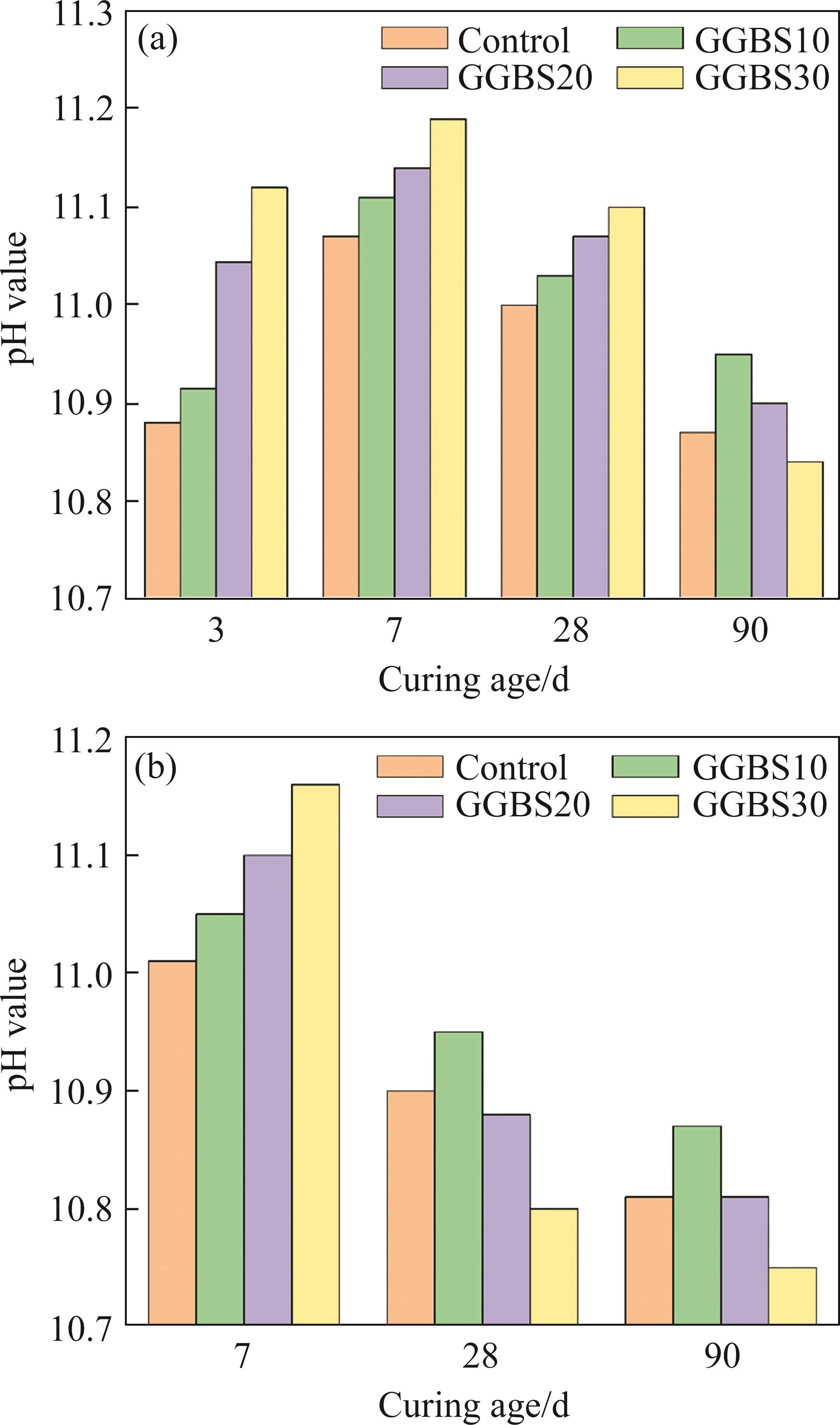
When GGBS exhibits the pozzolanic effect, it consumes CH, resulting in very limited CH formation within the paste, which results in only a minor change in the overall pH value. Additionally, an increase in GGBS content results in a rapid reduction in the pH value. Samples undergoing curing in the seawater generally exhibit lower pH values compared to those cured in tap water. The presence of Mg2+ and Ca2+ ions in the seawater leads to the adsorption of OH- ions within the pore solution, thereby inducing a decrease in the pH value. In general, AFt demonstrates stability when the pH value remains above 10.7 [6], but the pH value of samples cured in seawater continues to decrease, possibly suggesting that prolonged exposure to seawater during the curing period is not conducive to the stability of AFt, this is consistent with the XRD pattern in Section 3.2. It is important to emphasize that throughout the testing procedure, the pH value of the pore solution inevitably experiences a decline owing to carbonation resulting from air exposure [45].
3.7 Pore structure
When concrete is exposed to seawater, the accumulation of salts in the pores leads to physical damage to the matrix. When the pores are filled with salt crystals, the salts transfer stress to the pore walls, which is extremely detrimental to the durability of concrete [46]. This section analyzes the pore structure of samples cured in seawater for 90 d. Figure 13 shows the differential pore size distributions and porosities of different samples. The peak pore size in the derivative curve for both the control sample and the sample with 20% GGBS content is centered around 10 nm. Figure 14 illustrates the pore volume distribution of different samples cured in seawater, categorized into gel pores (less than 10 nm), small capillary pores (10 nm-50 nm), medium capillary pores (50 nm-100 nm), submicron large pores (100 nm-1000 nm), micron large pores (1-10 µm), and macropores (above 10 μm). These pore classifications are commonly observed in cement pastes [47]. It should be noted that the test results may overestimate the quantity of small pores due to the inherent accessibility issues (ink-bottle effect) in MIP testing [48].
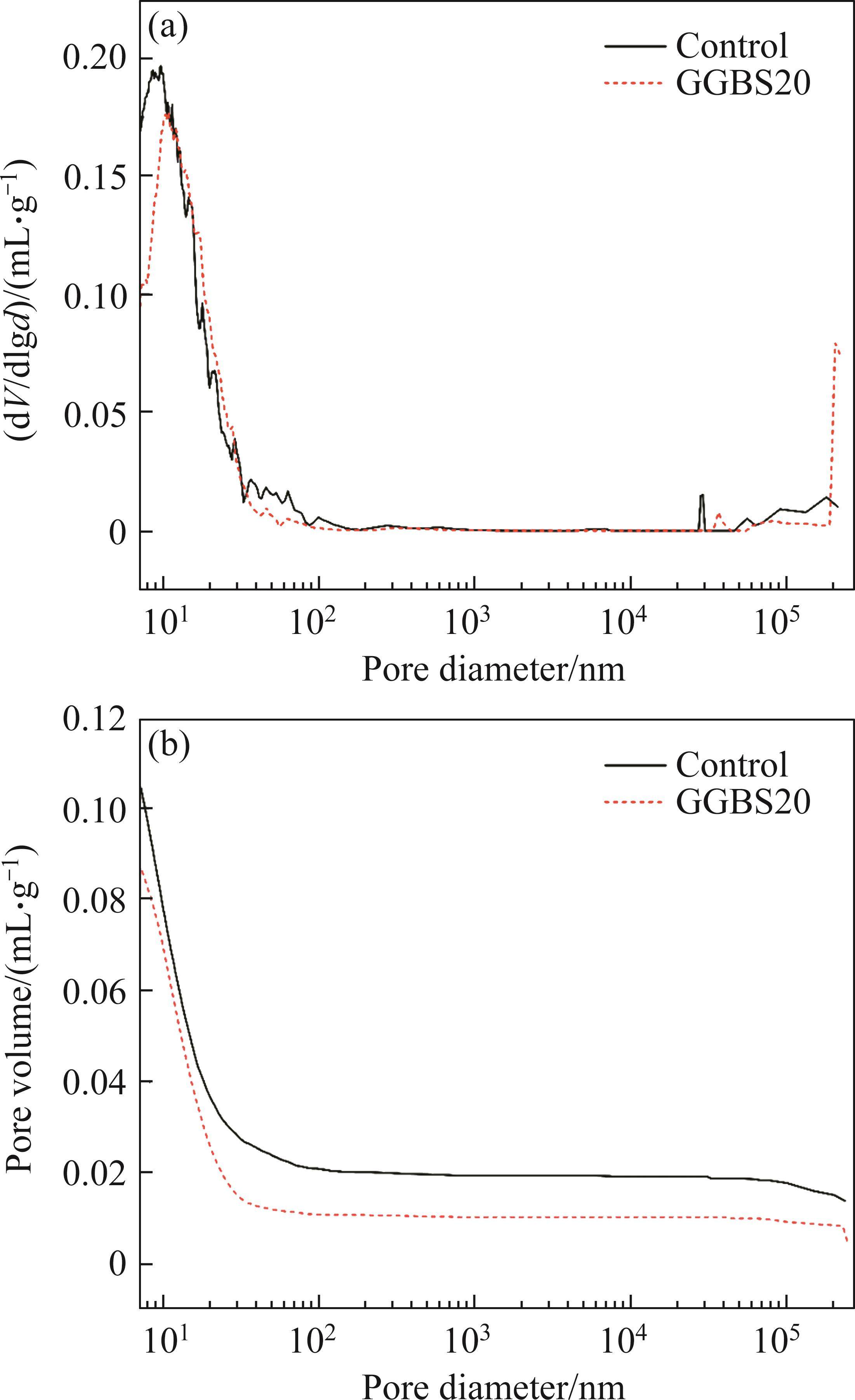
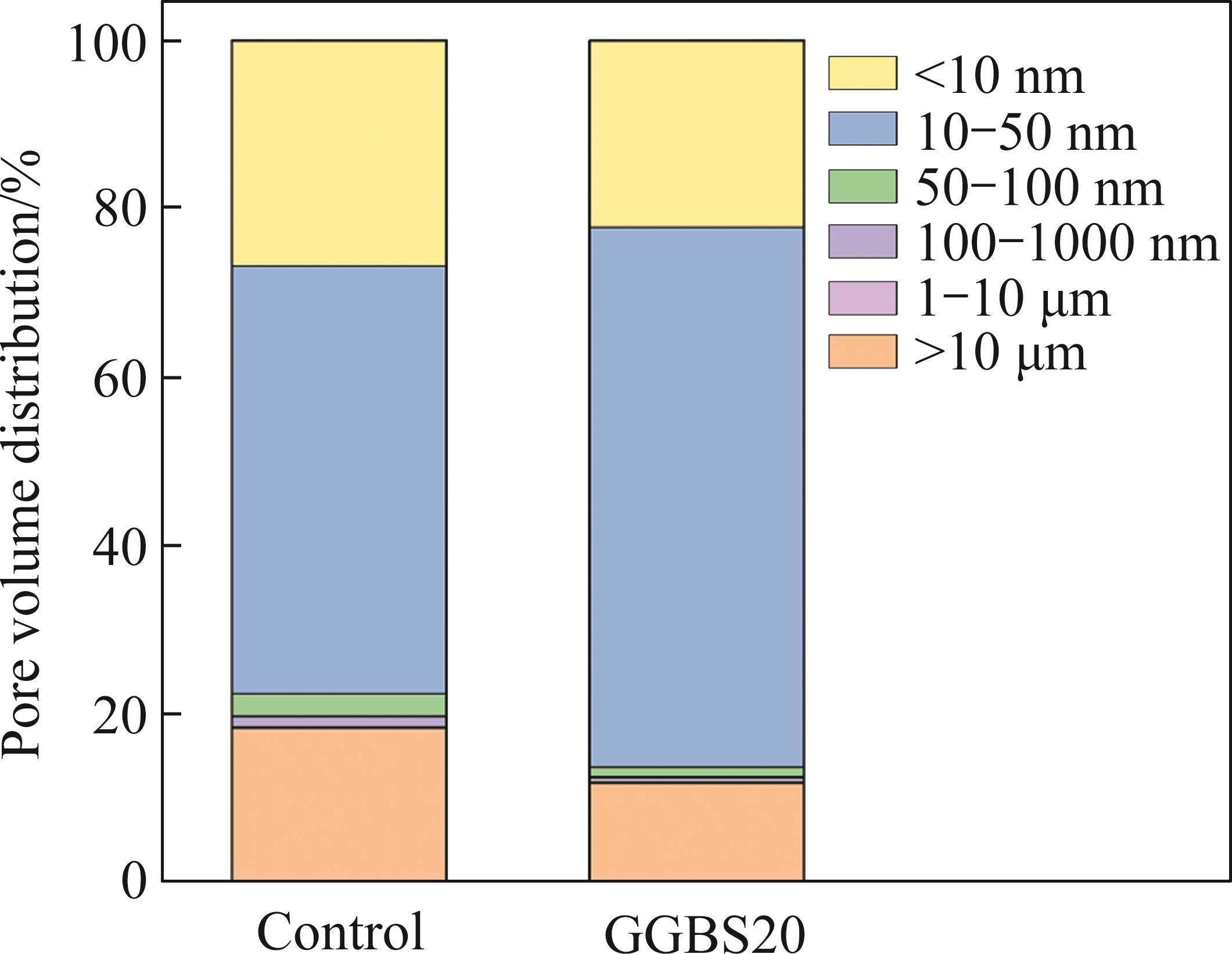
Pores with diameters larger than 50 nm are considered harmful pores, as they have a significant impact on the performance of concrete [47]. The total porosities of the two samples are 18.78% and 14.98%, respectively, with the proportion of pores larger than 50 nm being 22.4% and 13.65%, respectively. It can be observed that GGBS reduces the total porosity and the proportion of harmful pores in the control sample by 3.8% and 8.75%, respectively. Mineral admixtures are often added to cement to promote the consumption of CH, thereby reducing the porosity and achieving a uniform pore distribution in the cement matrix [49]. However, in the XRD patterns of the hydration products shown in Figure 9, no CH is detected. From the particle size distribution shown in Figure 3, it can be seen that GGBS generally has smaller particle sizes than FCSA cement. Compared to FCSA cement, the particle size distribution of GGBS is concentrated in the range of smaller than 100 µm. Therefore, it is possible that the filling effect of mineral admixtures effectively reduces the total porosity, fills and refines the pores and capillary pores in the hardened cement paste, and reduces the proportion of harmful pores [50]. AFt has a significant influence on the strength and durability development of concrete [39], the main hydration product of FCSA cement pastes, AFt, generates smaller pores (less than 10 nm). Compared to the control sample, the sample with GGBS has a lower proportion of pores smaller than 10 nm, which may indicate the formation of few AFt or gel-like substances.
4 Conclusions
In this study, the contrasting performance of specimens in tap water and seawater was investigated, with a primary focus on the influence of GGBS on the hydration properties of FCSA cement paste. Cement pastes were prepared with GGBS substitutions of 0%, 10%, 20%, and 30% in place of FCSA cement, maintaining a water-to-binder ratio of 0.45. The hydration process and evolution of hydration products in the cement pastes were characterized using hydration heat, XRD, and TG-DTG analysis. The main areas of assessment included the mechanical performance of FCSA cement pastes in both tap water and seawater, as well as the changes in specimen mass observed in seawater. Additionally, pH and MIP analyses were conducted to examine the pore solution and pore structure of the specimens. The following conclusions were drawn:
1) FCSA cement exhibits a fast early-age hydration rate and concentrated heat release. The addition of GGBS reduces the overall heat release of FCSA cement pastes and, to some extent, promotes the formation of AFt.
2) The compressive strength of FCSA cement paste demonstrates continuous growth over time. However, the presence of a low alkaline hydration environment diminishes the reactivity of GGBS, leading to reduced compressive strength in the specimens. Seawater curing, compared to tap water, enhances compressive strength and supports partial hydration of aluminum phases within GGBS. Furthermore, the influence of seawater on pH levels is evident, with the addition of GGBS affecting early and later-stage pH values. Seawater-cured samples experience gradual mass accumulation due to salt content, particularly in systems with higher GGBS content. This proportionality influences the rate of mass change in the system.
3) XRD and TG results indicate that the primary hydration products in FCSA cement pastes are AFt, FH3, and AH3. AFt exhibits significant early-stage generation, followed by a slight increase in later stages. Prolonged exposure of samples to seawater leads to partial hydrolysis of AFt. The AFm phase in FCSA cement reacts with Cl- in seawater, resulting in the formation of Friedel’s salt. In cement pastes containing 20% GGBS, there is a reduction in the quantity of hydration products, and their temporal evolution follows a pattern similar to that of pure cement pastes when exposed to seawater.
4) The coefficient of corrosion of the specimens remains consistent within the range of 1 to 1.3. The integration of GGBS promotes the compactness of the binder material, effectively retarding the formation of expansive products like gypsum and ettringite. This augmentation consequently elevates the coefficient of corrosion. GGBS as a filler refines the pore structure, diminishing porosity and the proportion of detrimental pores (>50 nm).
Durability and micromechanical properties of biochar in biochar-cement composites under marine environment
[J]. Journal of Cleaner Production, 2024, 450: 141842. DOI: 10.1016/j.jclepro.20 24.141842.Durability deterioration of concrete under marine environment from material to structure: A critical review
[J]. Journal of Building Engineering, 2021, 35: 102074. DOI: 10.1016/j.jobe.2020.102074.Effects of cement type, water/cement ratio and cement content on sea water resistance of concrete
[J]. Building and Environment, 2007, 42(4): 1770-1776. DOI: 10.1016/j.buildenv.2006.01.008.Mechanism of Friedel’s salt formation in cements rich in tri-calcium aluminate
[J]. Cement and Concrete Research, 1996, 26(5): 717-727. DOI: 10.1016/S0008-8846(96)85009-5.Friedel’s salt, Ca2Al(OH)6(Cl, OH)·2H2O: Its solid solutions and their role in chloride binding
[J]. Cement and Concrete Research, 1998, 28(12): 1713-1723. DOI: 10.1016/S0008-8846(98)001 62-8.Stability and solubility relationships in AFm phases
[J]. Cement and Concrete Research, 1999, 29(6): 861-866. DOI: 10.1016/s0008-8846(99)00055-1.A review on the deterioration and approaches to enhance the durability of concrete in the marine environment
[J]. Cement and Concrete Composites, 2020, 113: 103695. DOI: 10.1016/j.cemconcomp.2020.103695.Degradation mechanisms of Portland cement mortar under seawater attack and drying-wetting cycles
[J]. Construction and Building Materials, 2020, 230: 116934. DOI: 10.1016/j.conbuildmat.2019.116934.Effects of slag on the degradation mechanism of ordinary Portland cement-calcium aluminate cement-gypsum ternary binder under the multiple erosive ions
[J]. Construction and Building Materials, 2022, 324: 126661. DOI: 10.1016/j.conbuildmat. 2022.126661.Study on properties and degradation mechanism of calcium sulphoaluminate cement-ordinary Portland cement binary repair material under seawater erosion
[J]. Case Studies in Construction Materials, 2022, 17:Ferritic calcium sulfoaluminate belite cement from metallurgical industry residues and phosphogypsum: Clinker production, scale-up, and microstructural characterisation
[J]. Cement and Concrete Research, 2022, 154: 106715. DOI: 10.1016/j.cemconres.2022.106715.Understanding the role of brownmilerite on corrosion resistance
[J]. Construction and Building Materials, 2020, 254: 119262. DOI: 10.1016/j.conbuildmat.2020.119262.Study on the preparation and sulfate resistance of Portland cement clinker with the high Fe/Al ratio of ferrite phase
[J]. Cement and Concrete Composites, 2022, 134: 104699. DOI: 10.1016/j.cemconcomp.2022.104699.Enhanced sulfate resistance: The importance of iron in aluminate hydrates
[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(7): 6792-6801. DOI: 10.1021/acssuschemeng.8b06097.Constituent phases optimization of modified sulphoaluminate cement and its characteristic of Cl- solidification and resistance to marine erosion
[J]. Construction and Building Materials, 2021, 311: 125320. DOI: 10.1016/j.conbuildmat. 2021.125320.Exploration of hydration and durability properties of ferroaluminate cement with compare to Portland cement
[J]. Construction and Building Materials, 2022, 319: 126138. DOI: 10.1016/j.conbuildmat.2021.126138.Effects of different composite mineral admixtures on the early hydration and long-term properties of cement-based materials: A comparative study
[J]. Construction and Building Materials, 2021, 294: 123547. DOI: 10.1016/j.conbuildmat.2021.123547.Comparative research on the effect of various mineral admixtures on the early hydration process of cement
[J]. Construction and Building Materials, 2021, 301: 124372. DOI: 10.1016/j.conbuildmat.2021.124372.Effect of mineral admixtures on the structural build-up of cement paste
[J]. Construction and Building Materials, 2018, 160: 117-126. DOI: 10.1016/j.conbuildmat.2017.11.050.The relationship between autogenous shrinkage and pore structure of cement paste with mineral admixtures
[J]. Construction and Building Materials, 2010, 24(10): 1855-1860. DOI: 10.1016/j.conbuildmat.2010.04.018.Influence of metakaolin on pore structure-related properties and thermodynamic stability of hydrate phases of concrete in seawater environment
[J]. Construction and Building Materials, 2012, 36: 947-953. DOI: 10.1016/j.conbuildmat. 2012.06.073.Effects of mineral and chemical admixtures on high-strength concrete in seawater
[J]. Cement and Concrete Research, 2002, 32(3): 373-377. DOI: 10.1016/S0008-8846(01)00687-1.Durability and strength evaluation of high-performance concrete in marine structures
[J]. Construction and Building Materials, 2010, 24(6): 878-884. DOI: 10.1016/j.conbuildmat.2010.01.013.Performance of seawater-mixed concrete in the tidal environment
[J]. Cement and Concrete Research, 2004, 34(4): 593-601. DOI: 10.1016/j.cemconres.2003.09.020.Effect of brick powder on the pore solution and microstructure of Portland cement
[J]. Journal of Building Engineering, 2023, 63: 105497. DOI: 10.1016/j.jobe.2022.105497.Thermal control effects and mechanism of slag and fly ash on heat development of cement slurry used in hydrate formation
[J]. Journal of Natural Gas Science and Engineering, 2021, 91: 103967. DOI: 10.1016/j.jngse.2021.103967.Comparison on early hydration of Portland cement and sulphoaluminate cement in the presence of nano ettringite
[J]. Construction and Building Materials, 2022, 360: 129516. DOI: 10.1016/j.conbuildmat.2022.129516.Shrinkage behaviour, early hydration and hardened properties of sodium silicate activated slag incorporated with gypsum and cement
[J]. Construction and Building Materials, 2020, 248: 118687. DOI: 10.1016/j.conbuildmat.2020.118687.Early hydration heat of calcium sulfoaluminate cement with influences of supplementary cementitious materials and water to binder ratio
[J]. Materials, 2021, 14(3): 642. DOI: 10.3390/ma1403 0642.Chemical shrinkage of ferrite-rich calcium sulfoaluminate clinkers with varied gypsum contents
[J]. Construction and Building Materials, 2022, 357: 128729. DOI: 10.1016/j.conbuildmat.2022.128729.The effect of seawater on the phase assemblage of hydrated cement paste: A study on PC, CAC and CSA
[J]. Developments in the Built Environment, 2023, 15: 100183. DOI: 10.1016/j.dibe.2023. 100183.Friedel’s salt formation in sulfoaluminate cements: A combined XRD and 27Al MAS NMR study
[J]. Cement and Concrete Research, 2015, 67: 93-102. DOI: 10.1016/j.cemconres.2014.08.004.Production and properties of ferrite-rich CSAB cement from metallurgical industry residues
[J]. Science of the Total Environment, 2020, 712: 136208. DOI: 10.1016/j.scitotenv.2019.136208.Seawater resistance of blastfurnace slag activated by reactive magnesia with different reactivities: Durability performance and deterioration mechanism
[J]. Construction and Building Materials, 2024, 444: 137832. DOI: 10.1016/j. conbuildmat. 2024.137832.Long-term performance of ferrite-rich calcium sulfoaluminate cement-based paste under seawater corrosion
[J]. Construction and Building Materials, 2023, 377: 131056. DOI: 10.1016/j.conbuildmat.2023.131056.Behavior of high performance concrete pastes with different mineral admixtures in simulated seawater environment
[J]. Construction and Building Materials, 2018, 187: 426-438. DOI: 10.1016/j.conbuildmat.2018.07.196.Modeling ion and fluid transport in unsaturated cement systems in isothermal conditions
[J]. Cement and Concrete Research, 2005, 35(1): 141-153. DOI: 10.1016/j.cemconres. 2004.07.016.Modeling the three-dimensional unsaturated water transport in concrete at the mesoscale
[J]. Computers & Structures, 2017, 190: 61-74. DOI: 10.1016/j.compstruc.2017.05.005.The effect of seawater curing on properties of magnesium potassium phosphate cement
[J]. Construction and Building Materials, 2017, 141: 470-478. DOI: 10.1016/j.conbuildmat. 2017.02.057.Formation and function of ettringite in cement hydrates
[J]. Journal of the Chinese Ceramic Society, 2017, 45(11): 1569-1581. DOI: 10.14062/j.issn.0454-5648.2017.11.04.Study on the resistance to seawater corrosion of the cementitious systems containing ordinary Portland cement or/and calcium aluminate cement
[J]. Construction and Building Materials, 2017, 157: 852-859. DOI: 10.1016/j.conbuildmat.2017. 09.175.Properties evolution of calcium sulfoaluminate cement blended with ground granulated blast furnace slag suffered from sulfate attack
[J]. Journal of Materials Research and Technology, 2022, 17: 1642-1651. DOI: 10.1016/j.jmrt.2022.01.133.Hydration of calcium sulfoaluminate cements—Experimental findings and thermodynamic modelling
[J]. Cement and Concrete Research, 2010, 40(8): 1239-1247. DOI: 10.1016/j.cemconres.2009.08.014.The effect of a Pfa with a high total alkali content on pore solution composition and alkali silica reaction
[J]. Magazine of Concrete Research, 1986, 38(134): 30-35. DOI: 10.1680/macr.1986.38.134.30.Influence of limestone on the hydration of Portland cements
[J]. Cement and Concrete Research, 2008, 38(6): 848-860. DOI: 10.1016/j.cemconres.2008.01.002.Role of pore structure on resistance to physical crystallization damage of calcium sulfoaluminate belite (CSAB) cement blends
[J]. Cement and Concrete Research, 2022, 159: 106886. DOI: 10.1016/j.cemconres.2022.106886.Pore structure and chloride permeability of concrete containing nano-particles for pavement
[J]. Construction and Building Materials, 2011, 25(2): 608-616. DOI: 10.1016/j.conbuildmat.2010.07.032.Ink-bottle effect and pore size distribution of cementitious materials identified by pressurization-depressurization cycling mercury intrusion porosimetry
[J]. Materials, 2019, 12(9): 1454. DOI: 10.3390/ma12091454.Micro-nano scale pore structure and fractal dimension of ultra-high performance cementitious composites modified with nanofillers
[J]. Cement and Concrete Composites, 2023, 141: 105129. DOI: 10.1016/j.cemconcomp.2023.105129.Fly ash effects III. The microaggregate effect of fly ash
[J]. Cement and Concrete Research, 2004, 34(11): 2061-2066. DOI: 10.1016/j.cemconres.2003.03.002.CHEN Jia-wen, LIAO Yi-shun, MA Feng and TANG Sheng-wen declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CHEN Jia-wen, LIAO Yi-shun, MA Feng, TANG Sheng-wen. Effect of ground granulated blast furnace slag on hydration characteristics of ferrite-rich calcium sulfoaluminate cement in seawater [J]. Journal of Central South University, 2025, 32(1): 189-204. DOI: https://doi.org/10.1007/s11771-024-5794-1.
陈佳文,廖宜顺,马丰等.海水环境下矿渣对高铁硫铝酸盐水泥水化特性的影响[J].中南大学学报(英文版),2025,32(1):189-204.

